Clinical experience with temsirolimus in the treatment of advanced renal cell carcinoma
- PMID: 26161146
- PMCID: PMC4485412
- DOI: 10.1177/1756287215574457
Clinical experience with temsirolimus in the treatment of advanced renal cell carcinoma
Abstract
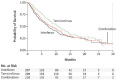
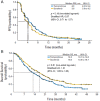
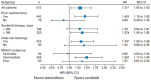
Temsirolimus is an inhibitor of the mammalian target of rapamycin (mTOR) kinase, a protein that has been shown to be particularly active in metastatic renal cell carcinoma (mRCC) with poor prognosis. Therefore, temsirolimus should be considered as the first-line treatment indicated in mRCC patients classified as poor risk. The benefits of temsirolimus are not limited to an increased survival but are also related to a better quality of life, which is certainly one of the most important aspects in the clinical management of these frail patients. Temsirolimus is a well-tolerated treatment, and the most frequent adverse events are manageable with supportive care. To this end, the identification of predictive factors of response to temsirolimus could help us to better select patients and obtain a more tailored clinical management of mRCC.
Keywords: mTOR inhibitors; renal cell carcinoma; targeted therapies; temsirolimus.
Conflict of interest statement
Figures

Similar articles
-
First-line Mammalian target of rapamycin inhibition in metastatic renal cell carcinoma: an analysis of practice patterns from the International Metastatic Renal Cell Carcinoma Database Consortium.Clin Genitourin Cancer. 2014 Oct;12(5):335-40. doi: 10.1016/j.clgc.2014.03.003. Epub 2014 Mar 15. Clin Genitourin Cancer. 2014. PMID: 24787966 Free PMC article.
-
Temsirolimus for advanced renal cell carcinoma.Expert Rev Anticancer Ther. 2014 Jan;14(1):9-21. doi: 10.1586/14737140.2014.864562. Epub 2013 Dec 6. Expert Rev Anticancer Ther. 2014. PMID: 24313573
-
Everolimus vs. temsirolimus for advanced renal cell carcinoma: use and use of resources in the US Oncology Network.Clin Genitourin Cancer. 2013 Jun;11(2):115-20. doi: 10.1016/j.clgc.2012.09.008. Epub 2012 Oct 12. Clin Genitourin Cancer. 2013. PMID: 23063578
-
Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma.Semin Oncol. 2009 Dec;36 Suppl 3:S26-36. doi: 10.1053/j.seminoncol.2009.10.013. Semin Oncol. 2009. PMID: 19963097 Review.
-
mTOR-inhibition in metastatic renal cell carcinoma. Focus on temsirolimus: a review.Minerva Urol Nefrol. 2010 Dec;62(4):411-23. Minerva Urol Nefrol. 2010. PMID: 20944541 Review.
Cited by
-
mTOR Signaling: Recent Progress.Int J Mol Sci. 2024 Feb 23;25(5):2587. doi: 10.3390/ijms25052587. Int J Mol Sci. 2024. PMID: 38473834 Free PMC article.
-
Characterization of the Prognosis and Tumor Microenvironment of Cellular Senescence-related Genes through scRNA-seq and Bulk RNA-seq Analysis in GC.Recent Pat Anticancer Drug Discov. 2024;19(4):530-542. doi: 10.2174/0115748928255417230924191157. Recent Pat Anticancer Drug Discov. 2024. PMID: 37807645
-
Recent Advances and Challenges of mTOR Inhibitors Use in the Treatment of Patients with Tuberous Sclerosis Complex.Oxid Med Cell Longev. 2017;2017:9820181. doi: 10.1155/2017/9820181. Epub 2017 Mar 12. Oxid Med Cell Longev. 2017. PMID: 28386314 Free PMC article. Review.
-
mTOR function and therapeutic targeting in breast cancer.Am J Cancer Res. 2017 Mar 1;7(3):383-404. eCollection 2017. Am J Cancer Res. 2017. PMID: 28400999 Free PMC article. Review.
-
Synergistic Activity of Paclitaxel, Sorafenib, and Radiation Therapy in advanced Renal Cell Carcinoma and Breast Cancer.Transl Oncol. 2019 Feb;12(2):381-388. doi: 10.1016/j.tranon.2018.11.007. Epub 2018 Dec 3. Transl Oncol. 2019. PMID: 30522045 Free PMC article.
References
-
- Atkins M., Hidalgo M., Stadler W., Logan T., Dutcher J., Hudes G., et al. (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22: 909–918. - PubMed
-
- Bellmunt J., Szczylik C., Feingold J., Strahs A., Berkenblit A. (2008) Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Ann Oncol 19: 1387–1392. - PubMed
-
- Bukowski R., Negrier S., Elson P. (2004) Prognostic factors in patients with advanced renal cell carcinoma: development of an international kidney cancer working group. Clin Cancer Res 10: 6310S–6314S. - PubMed
-
- Cheville J., Lohse C., Zincke H., Weaver A., Blute M. (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27: 612–624. - PubMed
-
- Cohen H., McGovern F. (2005) Renal-cell carcinoma. N Engl J Med 353: 2477–2490. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

