Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders
- PMID: 26161384
- PMCID: PMC4479819
- DOI: 10.3389/fcell.2015.00040
Dysfunctional mitochondrial bioenergetics and the pathogenesis of hepatic disorders
Abstract
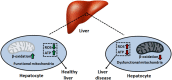
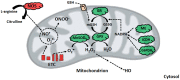
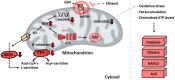
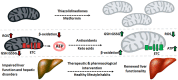
The liver is involved in a variety of critical biological functions including the homeostasis of glucose, fatty acids, amino acids, and the synthesis of proteins that are secreted in the blood. It is also at the forefront in the detoxification of noxious metabolites that would otherwise upset the functioning of the body. As such, this vital component of the mammalian system is exposed to a notable quantity of toxicants on a regular basis. It therefore comes as no surprise that there are over a hundred disparate hepatic disorders, encompassing such afflictions as fatty liver disease, hepatitis, and liver cancer. Most if not all of liver functions are dependent on energy, an ingredient that is primarily generated by the mitochondrion, the power house of all cells. This organelle is indispensable in providing adenosine triphosphate (ATP), a key effector of most biological processes. Dysfunctional mitochondria lead to a shortage in ATP, the leakage of deleterious reactive oxygen species (ROS), and the excessive storage of fats. Here we examine how incapacitated mitochondrial bioenergetics triggers the pathogenesis of various hepatic diseases. Exposure of liver cells to detrimental environmental hazards such as oxidative stress, metal toxicity, and various xenobiotics results in the inactivation of crucial mitochondrial enzymes and decreased ATP levels. The contribution of the latter to hepatic disorders and potential therapeutic cues to remedy these conditions are elaborated.
Keywords: energy; hepatocytes; liver disorders; metabolism; mitochondrial dysfunction.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

