Molecular Profiling-Selected Therapy for Treatment of Advanced Pancreaticobiliary Cancer: A Retrospective Multicenter Study
- PMID: 26161408
- PMCID: PMC4464000
- DOI: 10.1155/2015/681653
Molecular Profiling-Selected Therapy for Treatment of Advanced Pancreaticobiliary Cancer: A Retrospective Multicenter Study
Abstract
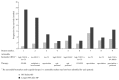
This multicenter cohort study assessed the impact of molecular profiling (MP) on advanced pancreaticobiliary cancer (PBC). The study included 30 patients treated with MP-guided therapy after failing ≥ 1 therapy for advanced PBC. Treatment was considered as having benefit for the patient if the ratio between the longest progression-free survival (PFS) on MP-guided therapy and the PFS on the last therapy before MP was ≥ 1.3. The null hypothesis was that ≤ 15% of patients gain such benefit. Overall, ≥ 1 actionable (i.e., predictive of response to specific therapies) biomarker was identified/patient. Immunohistochemistry (the most commonly used method for guiding treatment decisions) identified 1-6 (median: 4) actionable biomarkers per patient. After MP, patients received 1-4 (median: 1) regimens/patient (most commonly, FOLFIRI/XELIRI). In a decision-impact analysis, of the 27 patients for whom treatment decisions before MP were available, 74.1% experienced a treatment decision change in the first line after MP. Twenty-four patients were evaluable for clinical outcome analysis; in 37.5%, the PFS ratio was ≥ 1.3. In one-sided exact binomial test versus the null hypothesis, P = 0.0015; therefore, the null hypothesis was rejected. In conclusion, our analysis demonstrated the feasibility, clinical decision impact, and potential clinical benefits of MP-guided therapy in advanced PBC.
Figures
References
-
- NCCN. NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Version 1.2013. 2013. https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.or.... - PubMed
-
- Herrmann R., Bodoky G., Ruhstaller T., et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. Journal of Clinical Oncology. 2007;25(16):2212–2217. doi: 10.1200/jco.2006.09.0886. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical