Immediate Epileptogenesis after Kainate-Induced Status Epilepticus in C57BL/6J Mice: Evidence from Long Term Continuous Video-EEG Telemetry
- PMID: 26161754
- PMCID: PMC4498886
- DOI: 10.1371/journal.pone.0131705
Immediate Epileptogenesis after Kainate-Induced Status Epilepticus in C57BL/6J Mice: Evidence from Long Term Continuous Video-EEG Telemetry
Abstract
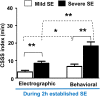
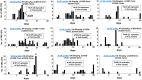
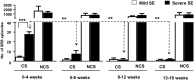
The C57BL/6J mouse as a model of seizure/epilepsy is challenging due to high mortality and huge variability in response to kainate. We have recently demonstrated that repeated administration of a low dose of kainate by intraperitoneal route can induce severe status epilepticus (SE) with 94% survival rate. In the present study, based on continuous video-EEG recording for 4-18 weeks from epidurally implanted electrodes on the cortex, we demonstrate that this method also induces immediate epileptogenesis (<1-5 days post-SE). This finding was based on identification of two types of spontaneous recurrent seizures; behavioral convulsive seizures (CS) and electrographic nonconvulsive seizures (NCS). The identification of the spontaneous CS, stage 3-5 types, was based on the behaviors (video) that were associated with the EEG characteristics (stage 3-5 epileptiform spikes), the power spectrum, and the activity counts. The electrographic NCS identification was based on the stage 1-2 epileptiform spike clusters on the EEG and their associated power spectrum. Severe SE induced immediate epileptogenesis in all the mice. The maximum numbers of spontaneous CS were observed during the first 4-6 weeks of the SE and they decreased thereafter. Mild SE also induced immediate epileptogenesis in some mice but the CS were less frequent. In both the severe and the mild SE groups, the spontaneous electrographic NCS persisted throughout the 18 weeks observation period, and therefore this could serve as a chronic model for complex seizures. However, unlike rat kainate models, the C57BL/6J mouse kainate model is a unique regressive CS model of epilepsy. Further studies are required to understand the mechanism of recovery from spontaneous CS in this model, which could reveal novel therapeutic targets for epilepsy.
Conflict of interest statement
Figures




Similar articles
-
Circadian clustering of spontaneous epileptic seizures emerges after pilocarpine-induced status epilepticus.Epilepsia. 2017 Jul;58(7):1159-1171. doi: 10.1111/epi.13795. Epub 2017 May 24. Epilepsia. 2017. PMID: 28542864
-
Immediate epileptogenesis: Impact on brain in C57BL/6J mouse kainate model.Front Biosci (Elite Ed). 2016 Jun 1;8(3):390-411. doi: 10.2741/e775. Front Biosci (Elite Ed). 2016. PMID: 27100347
-
Progression of convulsive and nonconvulsive seizures during epileptogenesis after pilocarpine-induced status epilepticus.J Neurophysiol. 2018 May 1;119(5):1818-1835. doi: 10.1152/jn.00721.2017. Epub 2018 Feb 14. J Neurophysiol. 2018. PMID: 29442558
-
Development of spontaneous seizures after experimental status epilepticus: implications for understanding epileptogenesis.Epilepsia. 2007;48 Suppl 5:157-63. doi: 10.1111/j.1528-1167.2007.01304.x. Epilepsia. 2007. PMID: 17910596 Review.
-
Status Epilepticus: Behavioral and Electroencephalography Seizure Correlates in Kainate Experimental Models.Front Neurol. 2018 Jan 23;9:7. doi: 10.3389/fneur.2018.00007. eCollection 2018. Front Neurol. 2018. PMID: 29410648 Free PMC article. Review.
Cited by
-
Soman-induced status epilepticus, epileptogenesis, and neuropathology in carboxylesterase knockout mice treated with midazolam.Epilepsia. 2018 Dec;59(12):2206-2218. doi: 10.1111/epi.14582. Epub 2018 Oct 25. Epilepsia. 2018. PMID: 30368799 Free PMC article.
-
Mechanisms of Excessive Extracellular Glutamate Accumulation in Temporal Lobe Epilepsy.Neurochem Res. 2017 Jun;42(6):1724-1734. doi: 10.1007/s11064-016-2105-8. Epub 2016 Nov 21. Neurochem Res. 2017. PMID: 27873132 Review.
-
Novel roles of ER stress in repressing neural activity and seizures through Mdm2- and p53-dependent protein translation.PLoS Genet. 2019 Sep 26;15(9):e1008364. doi: 10.1371/journal.pgen.1008364. eCollection 2019 Sep. PLoS Genet. 2019. PMID: 31557161 Free PMC article.
-
Inducible nitric oxide synthase inhibitor, 1400W, mitigates DFP-induced long-term neurotoxicity in the rat model.Neurobiol Dis. 2020 Jan;133:104443. doi: 10.1016/j.nbd.2019.03.031. Epub 2019 Mar 30. Neurobiol Dis. 2020. PMID: 30940499 Free PMC article.
-
The Kainic Acid Models of Temporal Lobe Epilepsy.eNeuro. 2021 Apr 9;8(2):ENEURO.0337-20.2021. doi: 10.1523/ENEURO.0337-20.2021. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33658312 Free PMC article. Review.
References
-
- Buckmaster PS. Laboratory animal models of temporal lobe epilepsy. Comp Med. 2004;54(5):473–85. Epub 2004/12/04. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

