Heme Stabilization of α-Synuclein Oligomers during Amyloid Fibril Formation
- PMID: 26161848
- PMCID: PMC4526360
- DOI: 10.1021/acs.biochem.5b00280
Heme Stabilization of α-Synuclein Oligomers during Amyloid Fibril Formation
Abstract
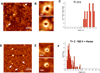
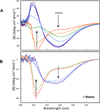
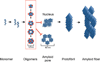
α-Synuclein (αSyn), which forms amyloid fibrils, is linked to the neuronal pathology of Parkinson's disease, as it is the major fibrillar component of Lewy bodies, the inclusions that are characteristic of the disease. Oligomeric structures, common to many neurodegenerative disease-related proteins, may in fact be the primary toxic species, while the amyloid fibrils exist either as a less toxic dead-end species or even as a beneficial mechanism for clearing damaged proteins. To alter the progression of the aggregation and gain insights into the prefibrillar structures, we determined the effect of heme on αSyn oligomerization by several different techniques, including native (nondenaturing) polyacrylamide gel electrophoresis, thioflavin T fluorescence, transmission electron microscopy, atomic force microscopy, circular dichroism, and membrane permeation using a calcein release assay. During aggregation, heme is able to bind the αSyn in a specific fashion, stabilizing distinct oligomeric conformations and promoting the formation of αSyn into annular structures, thereby delaying and/or inhibiting the fibrillation process. These results indicate that heme may play a regulatory role in the progression of Parkinson's disease; in addition, they provide insights into how the aggregation process may be altered, which may be applicable to the understanding of many neurodegenerative diseases.
Figures
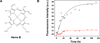




Similar articles
-
Physiological C-terminal truncation of α-synuclein potentiates the prion-like formation of pathological inclusions.J Biol Chem. 2018 Dec 7;293(49):18914-18932. doi: 10.1074/jbc.RA118.005603. Epub 2018 Oct 16. J Biol Chem. 2018. PMID: 30327435 Free PMC article.
-
Off-pathway oligomers of α-synuclein and Aβ inhibit secondary nucleation of α-synuclein amyloid fibrils.J Mol Biol. 2025 May 15;437(10):169048. doi: 10.1016/j.jmb.2025.169048. Epub 2025 Feb 25. J Mol Biol. 2025. PMID: 40015369
-
Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid.Biochemistry. 2000 Mar 14;39(10):2552-63. doi: 10.1021/bi991447r. Biochemistry. 2000. PMID: 10704204
-
Membrane interactions of oligomeric alpha-synuclein: potential role in Parkinson's disease.Curr Protein Pept Sci. 2010 Aug;11(5):334-42. doi: 10.2174/138920310791330659. Curr Protein Pept Sci. 2010. PMID: 20423294 Review.
-
Protein denaturation and aggregation: Cellular responses to denatured and aggregated proteins.Ann N Y Acad Sci. 2005 Dec;1066:181-221. doi: 10.1196/annals.1363.030. Ann N Y Acad Sci. 2005. PMID: 16533927 Review.
Cited by
-
Implications of COVID-19 in Parkinson's disease: the purinergic system in a therapeutic-target perspective to diminish neurodegeneration.Purinergic Signal. 2024 Oct;20(5):487-507. doi: 10.1007/s11302-024-09998-7. Epub 2024 Mar 9. Purinergic Signal. 2024. PMID: 38460075 Free PMC article. Review.
-
Mitochondrial Targeting in Neurodegeneration: A Heme Perspective.Pharmaceuticals (Basel). 2018 Sep 18;11(3):87. doi: 10.3390/ph11030087. Pharmaceuticals (Basel). 2018. PMID: 30231533 Free PMC article. Review.
-
Protein folding, misfolding and aggregation: The importance of two-electron stabilizing interactions.PLoS One. 2017 Sep 18;12(9):e0180905. doi: 10.1371/journal.pone.0180905. eCollection 2017. PLoS One. 2017. PMID: 28922400 Free PMC article.
-
An Analysis of the Multifaceted Roles of Heme in the Pathogenesis of Cancer and Related Diseases.Cancers (Basel). 2021 Aug 17;13(16):4142. doi: 10.3390/cancers13164142. Cancers (Basel). 2021. PMID: 34439295 Free PMC article. Review.
-
Pro-Oxidative and Inflammatory Actions of Extracellular Hemoglobin and Heme: Molecular Events and Implications for Alzheimer's and Parkinson Disease.Biomol Ther (Seoul). 2025 Mar 1;33(2):235-248. doi: 10.4062/biomolther.2024.224. Epub 2025 Feb 18. Biomol Ther (Seoul). 2025. PMID: 39962769 Free PMC article. Review.
References
-
- Glenner GG. Amyloid deposits and amyloidosis. The β-fibrilloses (first of two parts) N Engl J Med. 1980;302:1283–1292. - PubMed
-
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. - PubMed
-
- Goedert M. α-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. - PubMed
-
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. - PubMed
-
- Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM. Reversible inhibition of α-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem. 2005;280:21212–21219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

