Caspase-Cleaved Tau Co-Localizes with Early Tangle Markers in the Human Vascular Dementia Brain
- PMID: 26161867
- PMCID: PMC4498690
- DOI: 10.1371/journal.pone.0132637
Caspase-Cleaved Tau Co-Localizes with Early Tangle Markers in the Human Vascular Dementia Brain
Abstract
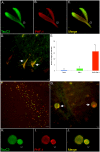
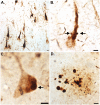
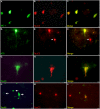
Vascular dementia (VaD) is the second most common form of dementia in the United States and is characterized as a cerebral vessel vascular disease that leads to ischemic episodes. Whereas the relationship between caspase-cleaved tau and neurofibrillary tangles (NFTs) in Alzheimer's disease (AD) has been previously described, whether caspase activation and cleavage of tau occurs in VaD is presently unknown. To investigate a potential role for caspase-cleaved tau in VaD, we analyzed seven confirmed cases of VaD by immunohistochemistry utilizing a well-characterized antibody that specifically detects caspase-cleaved tau truncated at Asp421. Application of this antibody (TauC3) revealed consistent labeling within NFTs, dystrophic neurites within plaque-rich regions and corpora amylacea (CA) in the human VaD brain. Labeling of CA by the TauC3 antibody was widespread throughout the hippocampus proper, was significantly higher compared to age matched controls, and co-localized with ubiquitin. Staining of the TauC3 antibody co-localized with MC-1, AT8, and PHF-1 within NFTs. Quantitative analysis indicated that roughly 90% of PHF-1-labeled NFTs contained caspase-cleaved tau. In addition, we documented the presence of active caspase-3 within plaques, blood vessels and pretangle neurons that co-localized with TauC3. Collectively, these data support a role for the activation of caspase-3 and proteolytic cleavage of TauC3 in VaD providing further support for the involvement of this family of proteases in NFT pathology.
Conflict of interest statement
Figures





Similar articles
-
Caspase-9 activation and caspase cleavage of tau in the Alzheimer's disease brain.Neurobiol Dis. 2002 Nov;11(2):341-54. doi: 10.1006/nbdi.2002.0549. Neurobiol Dis. 2002. PMID: 12505426
-
Apolipoprotein E pathology in vascular dementia.Int J Clin Exp Pathol. 2014 Feb 15;7(3):938-47. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 24696712 Free PMC article.
-
Characterization of TauC3 antibody and demonstration of its potential to block tau propagation.PLoS One. 2017 May 22;12(5):e0177914. doi: 10.1371/journal.pone.0177914. eCollection 2017. PLoS One. 2017. PMID: 28531180 Free PMC article.
-
The role of caspase cleavage of tau in Alzheimer disease neuropathology.J Neuropathol Exp Neurol. 2005 Feb;64(2):104-12. doi: 10.1093/jnen/64.2.104. J Neuropathol Exp Neurol. 2005. PMID: 15751224 Review.
-
Comparison between Alzheimer's disease and vascular dementia: implications for treatment.Neurol Res. 2003 Sep;25(6):661-4. doi: 10.1179/016164103101201968. Neurol Res. 2003. PMID: 14503021 Review.
Cited by
-
Caspase-cleaved tau is senescence-associated and induces a toxic gain of function by putting a brake on axonal transport.Mol Psychiatry. 2022 Jul;27(7):3010-3023. doi: 10.1038/s41380-022-01538-2. Epub 2022 Apr 7. Mol Psychiatry. 2022. PMID: 35393558 Free PMC article.
-
Carbonic Anhydrases as Potential Targets Against Neurovascular Unit Dysfunction in Alzheimer's Disease and Stroke.Front Aging Neurosci. 2021 Nov 16;13:772278. doi: 10.3389/fnagi.2021.772278. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34867298 Free PMC article. Review.
-
Impact of Tau on Neurovascular Pathology in Alzheimer's Disease.Front Neurol. 2021 Jan 7;11:573324. doi: 10.3389/fneur.2020.573324. eCollection 2020. Front Neurol. 2021. PMID: 33488493 Free PMC article. Review.
-
The Neuroanatomy of the Reticular Nucleus Locus Coeruleus in Alzheimer's Disease.Front Neuroanat. 2017 Sep 19;11:80. doi: 10.3389/fnana.2017.00080. eCollection 2017. Front Neuroanat. 2017. PMID: 28974926 Free PMC article. Review.
-
Astrocytes and neurons produce distinct types of polyglucosan bodies in Lafora disease.Glia. 2018 Oct;66(10):2094-2107. doi: 10.1002/glia.23463. Epub 2018 Aug 26. Glia. 2018. PMID: 30152044 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

