Quantitative Proteomic Analysis of BHK-21 Cells Infected with Foot-and-Mouth Disease Virus Serotype Asia 1
- PMID: 26161868
- PMCID: PMC4498813
- DOI: 10.1371/journal.pone.0132384
Quantitative Proteomic Analysis of BHK-21 Cells Infected with Foot-and-Mouth Disease Virus Serotype Asia 1
Abstract
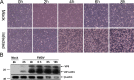
Stable isotope labeling with amino acids in cell culture (SILAC) was used to quantitatively study the host cell gene expression profile, in order to achieve an unbiased overview of the protein expression changes in BHK-21 cells infected with FMDV serotype Asia 1. The SILAC-based approach identified overall 2,141 proteins, 153 of which showed significant alteration in the expression level 6 h post FMDV infection (57 up-regulated and 96 down-regulated). Among these proteins, six cellular proteins, including three down-regulated (VPS28, PKR, EVI5) and three up-regulated (LYPLA1, SEC62 and DARs), were selected according to the significance of the changes and/or the relationship with PKR. The expression level and pattern of the selected proteins were validated by immunoblotting and confocal microscopy. Furthermore, the functions of these cellular proteins were assessed by small interfering RNA-mediated depletion, and their functional importance in the replication of FMDV was demonstrated by western blot, reverse transcript PCR (RT-PCR) and 50% Tissue Culture Infective Dose (TCID50). The results suggest that FMDV infection may have effects on the expression of specific cellular proteins to create more favorable conditions for FMDV infection. This study provides novel data that can be utilized to understand the interactions between FMDV and the host cell.
Conflict of interest statement
Figures






Similar articles
-
Quantitative proteomics by amino acid labeling in foot-and-mouth disease virus (FMDV)-infected cells.J Proteome Res. 2013 Jan 4;12(1):363-77. doi: 10.1021/pr300611e. Epub 2012 Nov 29. J Proteome Res. 2013. PMID: 23170859
-
Comparative Proteomic Analysis of Wild-Type and SAP Domain Mutant Foot-and-Mouth Disease Virus-Infected Porcine Cells Identifies the Ubiquitin-Activating Enzyme UBE1 Required for Virus Replication.J Proteome Res. 2015 Oct 2;14(10):4194-206. doi: 10.1021/acs.jproteome.5b00310. Epub 2015 Sep 21. J Proteome Res. 2015. PMID: 26354183
-
RNA interference targeting VP1 inhibits foot-and-mouth disease virus replication in BHK-21 cells and suckling mice.J Virol. 2004 Jul;78(13):6900-7. doi: 10.1128/JVI.78.13.6900-6907.2004. J Virol. 2004. PMID: 15194766 Free PMC article.
-
Cross-inhibition to heterologous foot-and-mouth disease virus infection induced by RNA interference targeting the conserved regions of viral genome.Virology. 2005 May 25;336(1):51-9. doi: 10.1016/j.virol.2005.01.051. Virology. 2005. PMID: 15866070
-
Using SILAC and quantitative proteomics to investigate the interactions between viral and host proteomes.Proteomics. 2012 Feb;12(4-5):666-72. doi: 10.1002/pmic.201100488. Epub 2012 Jan 19. Proteomics. 2012. PMID: 22246955 Review.
Cited by
-
Transcript Profiling Identifies Early Response Genes against FMDV Infection in PK-15 Cells.Viruses. 2018 Jul 11;10(7):364. doi: 10.3390/v10070364. Viruses. 2018. PMID: 29997306 Free PMC article.
-
Proof-of-concept study: profile of circulating microRNAs in Bovine serum harvested during acute and persistent FMDV infection.Virol J. 2017 Apr 7;14(1):71. doi: 10.1186/s12985-017-0743-3. Virol J. 2017. PMID: 28388926 Free PMC article.
-
ER-phagy: mechanisms, regulation, and diseases connected to the lysosomal clearance of the endoplasmic reticulum.Physiol Rev. 2022 Jul 1;102(3):1393-1448. doi: 10.1152/physrev.00038.2021. Epub 2022 Feb 21. Physiol Rev. 2022. PMID: 35188422 Free PMC article. Review.
-
CD44 mediates the internalization of foot-and-mouth disease virus through macropinocytosis.Vet Res. 2025 Jun 21;56(1):123. doi: 10.1186/s13567-025-01555-3. Vet Res. 2025. PMID: 40544280 Free PMC article.
-
Effect of different culture systems on the production of foot and mouth disease trivalent vaccine.Vet World. 2016 Jan;9(1):32-7. doi: 10.14202/vetworld.2016.32-37. Epub 2016 Jan 12. Vet World. 2016. PMID: 27051181 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

