Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation
- PMID: 26162324
- PMCID: PMC5109931
- DOI: 10.1007/s00395-015-0505-6
Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation
Abstract
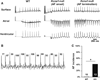
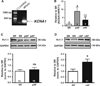
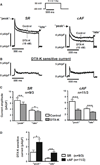
Voltage-gated Kv1.1 channels encoded by the Kcna1 gene are traditionally regarded as being neural-specific with no known expression or intrinsic functional role in the heart. However, recent studies in mice reveal low-level Kv1.1 expression in heart and cardiac abnormalities associated with Kv1.1-deficiency suggesting that the channel may have a previously unrecognized cardiac role. Therefore, this study tests the hypothesis that Kv1.1 channels are associated with arrhythmogenesis and contribute to intrinsic cardiac function. In intra-atrial burst pacing experiments, Kcna1-null mice exhibited increased susceptibility to atrial fibrillation (AF). The atria of Kcna1-null mice showed minimal Kv1 family ion channel remodeling and fibrosis as measured by qRT-PCR and Masson's trichrome histology, respectively. Using RT-PCR, immunocytochemistry, and immunoblotting, KCNA1 mRNA and protein were detected in isolated mouse cardiomyocytes and human atria for the first time. Patients with chronic AF (cAF) showed no changes in KCNA1 mRNA levels relative to controls; however, they exhibited increases in atrial Kv1.1 protein levels, not seen in paroxysmal AF patients. Patch-clamp recordings of isolated human atrial myocytes revealed significant dendrotoxin-K (DTX-K)-sensitive outward current components that were significantly increased in cAF patients, reflecting a contribution by Kv1.1 channels. The concomitant increases in Kv1.1 protein and DTX-K-sensitive currents in atria of cAF patients suggest that the channel contributes to the pathological mechanisms of persistent AF. These findings provide evidence of an intrinsic cardiac role of Kv1.1 channels and indicate that they may contribute to atrial repolarization and AF susceptibility.
Conflict of interest statement
Compliance with ethical standards Conflicts of interest None.
Figures



Similar articles
-
Genetic ablation or pharmacological inhibition of Kv1.1 potassium channel subunits impairs atrial repolarization in mice.Am J Physiol Cell Physiol. 2019 Feb 1;316(2):C154-C161. doi: 10.1152/ajpcell.00335.2018. Epub 2018 Nov 14. Am J Physiol Cell Physiol. 2019. PMID: 30427720 Free PMC article.
-
Expression of voltage-gated K+ channels in human atrium.Basic Res Cardiol. 2002 Nov;97(6):424-33. doi: 10.1007/s00395-002-0377-4. Basic Res Cardiol. 2002. PMID: 12395204
-
Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation.J Cardiovasc Electrophysiol. 2000 Jun;11(6):626-33. doi: 10.1111/j.1540-8167.2000.tb00024.x. J Cardiovasc Electrophysiol. 2000. PMID: 10868735
-
Kv1.1 channel subunits in the control of neurocardiac function.Channels (Austin). 2019 Dec;13(1):299-307. doi: 10.1080/19336950.2019.1635864. Channels (Austin). 2019. PMID: 31250689 Free PMC article. Review.
-
Atrial-Selective Potassium Channel Blockers.Card Electrophysiol Clin. 2016 Jun;8(2):411-21. doi: 10.1016/j.ccep.2016.02.005. Epub 2016 Mar 24. Card Electrophysiol Clin. 2016. PMID: 27261831 Review.
Cited by
-
Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation.Nat Rev Cardiol. 2016 Oct;13(10):575-90. doi: 10.1038/nrcardio.2016.118. Epub 2016 Aug 4. Nat Rev Cardiol. 2016. PMID: 27489190 Review.
-
Neuron-specific Kv1.1 deficiency is sufficient to cause epilepsy, premature death, and cardiorespiratory dysregulation.Neurobiol Dis. 2020 Apr;137:104759. doi: 10.1016/j.nbd.2020.104759. Epub 2020 Jan 21. Neurobiol Dis. 2020. PMID: 31978607 Free PMC article.
-
The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis.Cardiovasc Res. 2016 Apr 1;109(4):467-79. doi: 10.1093/cvr/cvv275. Epub 2015 Dec 23. Cardiovasc Res. 2016. PMID: 26705366 Free PMC article. Review.
-
Age-Related Differential Structural and Transcriptomic Responses in the Hypertensive Heart.Front Physiol. 2018 Jul 9;9:817. doi: 10.3389/fphys.2018.00817. eCollection 2018. Front Physiol. 2018. PMID: 30038575 Free PMC article.
-
Computational models of atrial cellular electrophysiology and calcium handling, and their role in atrial fibrillation.J Physiol. 2016 Feb 1;594(3):537-53. doi: 10.1113/JP271404. Epub 2015 Dec 28. J Physiol. 2016. PMID: 26582329 Free PMC article. Review.
References
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, Grandjean JG, Van Gilst WH, Crijns HJ. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–690. - PubMed
-
- Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, Wilde AA, Van Gilst WH, Crijns HJ. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol. 2001;37:926–932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

