Platelet binding sites for factor VIII in relation to fibrin and phosphatidylserine
- PMID: 26162408
- PMCID: PMC4559935
- DOI: 10.1182/blood-2015-01-620245
Platelet binding sites for factor VIII in relation to fibrin and phosphatidylserine
Abstract
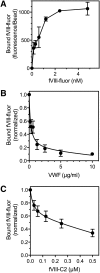
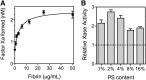
Thrombin-stimulated platelets expose very little phosphatidylserine (PS) but express binding sites for factor VIII (fVIII), casting doubt on the role of exposed PS as the determinant of binding sites. We previously reported that fVIII binding sites are increased three- to sixfold when soluble fibrin (SF) binds the αIIbβ3 integrin. This study focuses on the hypothesis that platelet-bound SF is the major source of fVIII binding sites. Less than 10% of fVIII was displaced from thrombin-stimulated platelets by lactadherin, a PS-binding protein, and an fVIII mutant defective in PS-dependent binding retained platelet affinity. Therefore, PS is not the determinant of most binding sites. FVIII bound immobilized SF and paralleled platelet binding in affinity, dependence on separation from von Willebrand factor, and mediation by the C2 domain. SF also enhanced activity of fVIII in the factor Xase complex by two- to fourfold. Monoclonal antibody (mAb) ESH8, against the fVIII C2 domain, inhibited binding of fVIII to SF and platelets but not to PS-containing vesicles. Similarly, mAb ESH4 against the C2 domain, inhibited >90% of platelet-dependent fVIII activity vs 35% of vesicle-supported activity. These results imply that platelet-bound SF is a component of functional fVIII binding sites.
Figures





Comment in
-
fVIII binds platelets + fibrin: no PS!Blood. 2015 Sep 3;126(10):1158-9. doi: 10.1182/blood-2015-07-657924. Blood. 2015. PMID: 26337352 Free PMC article.
Similar articles
-
Activation of factor VIII by thrombin increases its affinity for binding to synthetic phospholipid membranes and activated platelets.J Biol Chem. 1998 Oct 23;273(43):27918-26. doi: 10.1074/jbc.273.43.27918. J Biol Chem. 1998. PMID: 9774404
-
fVIII binds platelets + fibrin: no PS!Blood. 2015 Sep 3;126(10):1158-9. doi: 10.1182/blood-2015-07-657924. Blood. 2015. PMID: 26337352 Free PMC article.
-
The factor VIII C1 domain contributes to platelet binding.Blood. 2008 Jan 1;111(1):200-8. doi: 10.1182/blood-2007-01-068957. Epub 2007 Oct 4. Blood. 2008. PMID: 17916745 Free PMC article.
-
The evolving understanding of factor VIII binding sites and implications for the treatment of hemophilia A.Blood Rev. 2019 Jan;33:1-5. doi: 10.1016/j.blre.2018.05.001. Epub 2018 May 24. Blood Rev. 2019. PMID: 29866493 Review.
-
Role of activation of the coagulation factor VIII in interaction with vWf, phospholipid, and functioning within the factor Xase complex.Trends Cardiovasc Med. 1999 Oct;9(7):185-92. doi: 10.1016/s1050-1738(00)00019-0. Trends Cardiovasc Med. 1999. PMID: 10881749 Review.
Cited by
-
Fibrin Formation, Structure and Properties.Subcell Biochem. 2017;82:405-456. doi: 10.1007/978-3-319-49674-0_13. Subcell Biochem. 2017. PMID: 28101869 Free PMC article. Review.
-
The impact of von Willebrand factor on fibrin formation and structure unveiled with type 3 von Willebrand disease plasma.Blood Coagul Fibrinolysis. 2024 Jul 1;35(5):256-264. doi: 10.1097/MBC.0000000000001309. Epub 2024 May 20. Blood Coagul Fibrinolysis. 2024. PMID: 38973517 Free PMC article.
-
LDL receptor related protein 1 requires the I3 domain of discs-large homolog 1/DLG1 for interaction with the kinesin motor protein KIF13B.Biochim Biophys Acta Mol Cell Res. 2019 Dec;1866(12):118552. doi: 10.1016/j.bbamcr.2019.118552. Epub 2019 Sep 2. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31487503 Free PMC article.
-
The Central Role of Extracellular Vesicles in the Mechanisms of Thrombosis in COVID-19 Patients With Cancer and Therapeutic Strategies.Front Cell Dev Biol. 2022 Jan 12;9:792335. doi: 10.3389/fcell.2021.792335. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35096822 Free PMC article. Review.
-
Global coagulation assays in hemophilia A: A comparison to conventional assays.Res Pract Thromb Haemost. 2019 Dec 29;4(2):298-308. doi: 10.1002/rth2.12295. eCollection 2020 Feb. Res Pract Thromb Haemost. 2019. PMID: 32110761 Free PMC article.
References
-
- Gilbert GE, Furie BC, Furie B. Binding of human factor VIII to phospholipid vesicles. J Biol Chem. 1990;265(2):815–822. - PubMed
-
- Gilbert GE, Drinkwater D. Specific membrane binding of factor VIII is mediated by O-phospho-L-serine, a moiety of phosphatidylserine. Biochemistry. 1993;32(37):9577–9585. - PubMed
-
- Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76(1):1–16. - PubMed
-
- Nesheim M, Pittman DD, Giles AR, et al. The effect of plasma von Willebrand factor on the binding of human factor VIII to thrombin-activated human platelets. J Biol Chem. 1991;266(27):17815–17820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

