Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies
- PMID: 26162572
- PMCID: PMC5042203
- DOI: 10.1016/j.jaci.2015.05.036
Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies
Abstract
Background: Follicular helper T (TFH) cells underpin T cell-dependent humoral immunity and the success of most vaccines. TFH cells also contribute to human immune disorders, such as autoimmunity, immunodeficiency, and malignancy. Understanding the molecular requirements for the generation and function of TFH cells will provide strategies for targeting these cells to modulate their behavior in the setting of these immunologic abnormalities.
Objective: We sought to determine the signaling pathways and cellular interactions required for the development and function of TFH cells in human subjects.
Methods: Human primary immunodeficiencies (PIDs) resulting from monogenic mutations provide a unique opportunity to assess the requirement for particular molecules in regulating human lymphocyte function. Circulating follicular helper T (cTFH) cell subsets, memory B cells, and serum immunoglobulin levels were quantified and functionally assessed in healthy control subjects, as well as in patients with PIDs resulting from mutations in STAT3, STAT1, TYK2, IL21, IL21R, IL10R, IFNGR1/2, IL12RB1, CD40LG, NEMO, ICOS, or BTK.
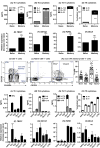
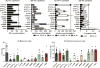
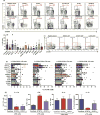
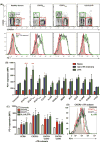
Results: Loss-of-function (LOF) mutations in STAT3, IL10R, CD40LG, NEMO, ICOS, or BTK reduced cTFH cell frequencies. STAT3 and IL21/R LOF and STAT1 gain-of-function mutations skewed cTFH cell differentiation toward a phenotype characterized by overexpression of IFN-γ and programmed death 1. IFN-γ inhibited cTFH cell function in vitro and in vivo, as corroborated by hypergammaglobulinemia in patients with IFNGR1/2, STAT1, and IL12RB1 LOF mutations.
Conclusion: Specific mutations affect the quantity and quality of cTFH cells, highlighting the need to assess TFH cells in patients by using multiple criteria, including phenotype and function. Furthermore, IFN-γ functions in vivo to restrain TFH cell-induced B-cell differentiation. These findings shed new light on TFH cell biology and the integrated signaling pathways required for their generation, maintenance, and effector function and explain the compromised humoral immunity seen in patients with some PIDs.
Keywords: Follicular helper T cells; cytokine signaling; humoral immunity; primary immunodeficiencies.
Copyright © 2015 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest
Figures


Similar articles
-
Functional STAT3 deficiency compromises the generation of human T follicular helper cells.Blood. 2012 Apr 26;119(17):3997-4008. doi: 10.1182/blood-2011-11-392985. Epub 2012 Mar 8. Blood. 2012. PMID: 22403255 Free PMC article.
-
Human T Follicular Helper Cells in Primary Immunodeficiency: Quality Just as Important as Quantity.J Clin Immunol. 2016 May;36 Suppl 1:40-7. doi: 10.1007/s10875-016-0257-6. Epub 2016 Mar 10. J Clin Immunol. 2016. PMID: 26961358 Review.
-
Aberrant Expansion and Function of Follicular Helper T Cell Subsets in IgG4-Related Disease.Arthritis Rheumatol. 2018 Nov;70(11):1853-1865. doi: 10.1002/art.40556. Epub 2018 Aug 29. Arthritis Rheumatol. 2018. PMID: 29781221 Free PMC article.
-
Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function.J Allergy Clin Immunol. 2013 Aug;132(2):400-11.e9. doi: 10.1016/j.jaci.2013.05.029. Epub 2013 Jul 4. J Allergy Clin Immunol. 2013. PMID: 23830147 Free PMC article.
-
Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses.Front Immunol. 2018 Oct 22;9:2412. doi: 10.3389/fimmu.2018.02412. eCollection 2018. Front Immunol. 2018. PMID: 30405612 Free PMC article. Review.
Cited by
-
Dominant TOM1 mutation associated with combined immunodeficiency and autoimmune disease.NPJ Genom Med. 2019 Jun 27;4:14. doi: 10.1038/s41525-019-0088-5. eCollection 2019. NPJ Genom Med. 2019. PMID: 31263572 Free PMC article.
-
Novel CD81 Mutations in a Chinese Patient Led to IgA Nephropathy and Impaired BCR Signaling.J Clin Immunol. 2022 Nov;42(8):1672-1684. doi: 10.1007/s10875-022-01333-2. Epub 2022 Jul 18. J Clin Immunol. 2022. PMID: 35849269
-
The Antigen Presenting Potential of CD21low B Cells.Front Immunol. 2020 Oct 21;11:535784. doi: 10.3389/fimmu.2020.535784. eCollection 2020. Front Immunol. 2020. PMID: 33193306 Free PMC article. Clinical Trial.
-
Transfer of gene-corrected T cells corrects humoral and cytotoxic defects in patients with X-linked lymphoproliferative disease.J Allergy Clin Immunol. 2018 Jul;142(1):235-245.e6. doi: 10.1016/j.jaci.2018.02.053. Epub 2018 Apr 27. J Allergy Clin Immunol. 2018. PMID: 29705247 Free PMC article.
-
Inborn errors of immunity reveal molecular requirements for generation and maintenance of human CD4+ IL-9-expressing cells.J Allergy Clin Immunol. 2025 Apr;155(4):1161-1178. doi: 10.1016/j.jaci.2024.11.031. Epub 2024 Nov 30. J Allergy Clin Immunol. 2025. PMID: 39622295
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

