Elastin governs the mechanical response of medial collateral ligament under shear and transverse tensile loading
- PMID: 26162584
- PMCID: PMC4629914
- DOI: 10.1016/j.actbio.2015.07.011
Elastin governs the mechanical response of medial collateral ligament under shear and transverse tensile loading
Abstract
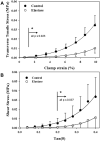
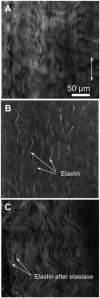
Elastin is a highly extensible structural protein network that provides near-elastic resistance to deformation in biological tissues. In ligament, elastin is localized between and along the collagen fibers and fascicles. When ligament is stretched along the primary collagen axis, elastin supports a relatively high percentage of load. We hypothesized that elastin may also provide significant load support under elongation transverse to the primary collagen axis and shear along the collagen axis. Quasi-static transverse tensile and shear material tests were performed to quantify the mechanical contributions of elastin during deformation of porcine medial collateral ligament. Dose response studies were conducted to determine the level of elastase enzymatic degradation required to produce a maximal change in the mechanical response. Maximal changes in peak stress occurred after 3h of treatment with 2U/ml porcine pancreatic elastase. Elastin degradation resulted in a 60-70% reduction in peak stress and a 2-3× reduction in modulus for both test protocols. These results demonstrate that elastin provides significant resistance to elongation transverse to the collagen axis and shear along the collagen axis while only constituting 4% of the tissue dry weight. The magnitudes of the elastin contribution to peak transverse and shear stress were approximately 0.03 MPa, as compared to 2 MPa for axial tensile tests, suggesting that elastin provides a highly anisotropic contribution to the mechanical response of ligament and is the dominant structural protein resisting transverse and shear deformation of the tissue.
Keywords: Elastase; Elastin; Ligament; Shear; Transverse tensile.
Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures



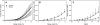

Similar articles
-
Effect of elastin digestion on the quasi-static tensile response of medial collateral ligament.J Orthop Res. 2013 Aug;31(8):1226-33. doi: 10.1002/jor.22352. Epub 2013 Mar 28. J Orthop Res. 2013. PMID: 23553827 Free PMC article.
-
Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament.J Orthop Res. 2007 Jul;25(7):894-903. doi: 10.1002/jor.20351. J Orthop Res. 2007. PMID: 17343278
-
Contributions of elastic fibers, collagen, and extracellular matrix to the multiaxial mechanics of ligament.J Mech Behav Biomed Mater. 2019 Nov;99:118-126. doi: 10.1016/j.jmbbm.2019.07.018. Epub 2019 Jul 20. J Mech Behav Biomed Mater. 2019. PMID: 31351401 Free PMC article.
-
Contributions of collagen and elastin to elastic behaviours of tendon fascicle.Acta Biomater. 2024 Mar 1;176:334-343. doi: 10.1016/j.actbio.2024.01.014. Epub 2024 Jan 17. Acta Biomater. 2024. PMID: 38237712
-
Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective.Biochim Biophys Acta. 2013 Jul;1832(7):866-75. doi: 10.1016/j.bbadis.2012.11.022. Epub 2012 Dec 6. Biochim Biophys Acta. 2013. PMID: 23220448 Review.
Cited by
-
Effect of detergent type on the performance of liver decellularized extracellular matrix-based bio-inks.J Tissue Eng. 2021 Feb 24;12:2041731421997091. doi: 10.1177/2041731421997091. eCollection 2021 Jan-Dec. J Tissue Eng. 2021. PMID: 33717429 Free PMC article.
-
Time-dependent extracellular matrix alterations of young tendons in response to stress relaxation: a model for the Ponseti method.J R Soc Interface. 2023 May;20(202):20220712. doi: 10.1098/rsif.2022.0712. Epub 2023 May 17. J R Soc Interface. 2023. PMID: 37194273 Free PMC article.
-
The mechanical response of the mouse cervix to tensile cyclic loading in term and preterm pregnancy.Acta Biomater. 2018 Sep 15;78:308-319. doi: 10.1016/j.actbio.2018.07.017. Epub 2018 Jul 29. Acta Biomater. 2018. PMID: 30059802 Free PMC article.
-
The anterior cruciate ligament in murine post-traumatic osteoarthritis: markers and mechanics.Arthritis Res Ther. 2022 May 30;24(1):128. doi: 10.1186/s13075-022-02798-7. Arthritis Res Ther. 2022. PMID: 35637500 Free PMC article.
-
Elastin is responsible for the rigidity of the ligament under shear and rotational stress: a mathematical simulation study.J Orthop Surg Res. 2023 Apr 19;18(1):310. doi: 10.1186/s13018-023-03794-6. J Orthop Surg Res. 2023. PMID: 37072855 Free PMC article.
References
-
- Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. - PubMed
-
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. - PubMed
-
- Gacko M. Elastin: Structure, properties, and metabolism. Cell & Mol Biol Lett. 2000;5:327–48.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

