Outcomes After Warfarin Initiation in a Cohort of Hemodialysis Patients With Newly Diagnosed Atrial Fibrillation
- PMID: 26162653
- PMCID: PMC4584203
- DOI: 10.1053/j.ajkd.2015.05.019
Outcomes After Warfarin Initiation in a Cohort of Hemodialysis Patients With Newly Diagnosed Atrial Fibrillation
Abstract
Background: Although warfarin is indicated to prevent ischemic strokes in most patients with atrial fibrillation (AF), evidence supporting its use in hemodialysis patients is limited. Our aim was to examine outcomes after warfarin therapy initiation, relative to no warfarin use, following incident AF in a large cohort of hemodialysis patients who had comprehensive prescription drug coverage through Medicare Part D.
Study design: Retrospective observational cohort study.
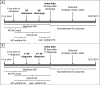
Setting & participants: Patients in the US Renal Data System undergoing maintenance hemodialysis who had AF newly diagnosed in 2007 to 2011, with Medicare Part D coverage, who had no recorded history of warfarin use.
Predictor: Warfarin therapy initiation, identified by a filled prescription within 30 days of the AF event.
Outcomes: Death, ischemic stroke, hemorrhagic stroke, severe gastrointestinal bleeding, and composite outcomes.
Measurements: HRs estimated by applying Cox regression to an inverse probability of treatment and censoring-weighted cohort.
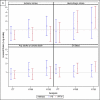
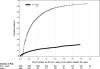
Results: Of 12,284 patients with newly diagnosed AF, 1,838 (15%) initiated warfarin therapy within 30 days; however, ∼70% discontinued its use within 1 year. In intention-to-treat analyses, warfarin use was marginally associated with a reduced risk of ischemic stroke (HR, 0.68; 95% CI, 0.47-0.99), but not with the other outcomes. In as-treated analyses, warfarin use was associated with reduced mortality (HR, 0.84; 95% CI, 0.73-0.97).
Limitations: Short observation period, limited number of nonfatal events, limited generalizability of results to more affluent patients.
Conclusions: In hemodialysis patients with incident AF, warfarin use was marginally associated with reduced risk of ischemic stroke, and there was a signal toward reduced mortality in as-treated analyses. These results support clinical equipoise regarding the use of warfarin in hemodialysis patients and underscore the need for randomized trials to fill this evidence gap.
Keywords: Dialysis; atrial fibrillation (AF); bleeding; cardiac arrhythmia; drug safety; end-stage renal disease (ESRD); hemodialysis; hemorrhagic stroke; ischemic stroke; mortality; oral anticoagulation; prevention; warfarin.
Copyright © 2015 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32DK007357/DK/NIDDK NIH HHS/United States
- K23 DK103972/DK/NIDDK NIH HHS/United States
- F32 DK096765/DK/NIDDK NIH HHS/United States
- KL2 TR000122/TR/NCATS NIH HHS/United States
- F32DK096765/DK/NIDDK NIH HHS/United States
- T32 DK007357/DK/NIDDK NIH HHS/United States
- K23 DK095914/DK/NIDDK NIH HHS/United States
- R21DK077336/DK/NIDDK NIH HHS/United States
- KL2TR000122/TR/NCATS NIH HHS/United States
- R01 DK095024/DK/NIDDK NIH HHS/United States
- K23DK095914/DK/NIDDK NIH HHS/United States
- R01DK095024/DK/NIDDK NIH HHS/United States
- R21 DK077336/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

