Changes in peripheral blood immune cell composition in osteoarthritis
- PMID: 26162804
- PMCID: PMC4638189
- DOI: 10.1016/j.joca.2015.06.018
Changes in peripheral blood immune cell composition in osteoarthritis
Abstract
Objectives: Immune age-related abnormalities may synergise with osteoarthritis (OA) pathology. We explored whether abnormalities in the blood immune cell composition are present in OA, beyond defects typically associated with ageing.
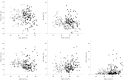
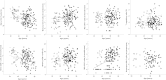
Design: Blood was collected from 121 healthy controls (HC) and 114 OA patients. Synovial biopsies were obtained from another 52 OA patients. Flow cytometry was used to establish the frequencies of lineage subsets, naïve, memory and regulatory T and B-cells, cells with an abnormal phenotype related to inflammation (IRC) and memory-like CD8(+) T-cells. Multivariate analysis of covariance (MANCOVA) was used to determine whether the relative subset frequencies differed between HC and OA, controlling for age.
Results: Expected histology and T/B-cell infiltration were observed. Following age adjusted analysis, we confirmed the lack of age association in HC for CD4(+), B, NK and NKT cells but a negative trend for CD8(+) T-cells. In OA, CD4(+) T-cell and B-cell frequency were lower compared to HC while CD8(+) T-cell frequencies were higher. CD8(+) memory-like cells were more likely to be found in OA (odds ratio = 15). Increased CD8(+) IRC frequencies were also present in OA. The relationship between age and CD4(+) or CD8(+) naïve T-cells in HC were changed in OA while the age relationships with memory cells were lost. The increase in CD4(+) Treg with age was also lost in OA. B-cells showed limited evidence of disturbance.
Conclusions: Immune dysfunction may be present in OA beyond what appears related to ageing; this requires further investigation.
Keywords: Ageing; Blood cell composition; Cell subsets/phenotype; OA.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

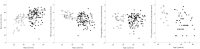
References
-
- Franceschi C., Bonafè M., Valensin S., Olivieri F., de Luca M., Ottaviani E. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. - PubMed
-
- Franceschi C., Bonafe M., Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

