Amphetamine activates calcium channels through dopamine transporter-mediated depolarization
- PMID: 26162812
- PMCID: PMC4631700
- DOI: 10.1016/j.ceca.2015.06.013
Amphetamine activates calcium channels through dopamine transporter-mediated depolarization
Abstract
Amphetamine (AMPH) and its more potent enantiomer S(+)AMPH are psychostimulants used therapeutically to treat attention deficit hyperactivity disorder and have significant abuse liability. AMPH is a dopamine transporter (DAT) substrate that inhibits dopamine (DA) uptake and is implicated in DA release. Furthermore, AMPH activates ionic currents through DAT that modify cell excitability presumably by modulating voltage-gated channel activity. Indeed, several studies suggest that monoamine transporter-induced depolarization opens voltage-gated Ca(2+) channels (CaV), which would constitute an additional AMPH mechanism of action. In this study we co-express human DAT (hDAT) with Ca(2+) channels that have decreasing sensitivity to membrane depolarization (CaV1.3, CaV1.2 or CaV2.2). Although S(+)AMPH is more potent than DA in transport-competition assays and inward-current generation, at saturating concentrations both substrates indirectly activate voltage-gated L-type Ca(2+) channels (CaV1.3 and CaV1.2) but not the N-type Ca(2+) channel (CaV2.2). Furthermore, the potency to achieve hDAT-CaV electrical coupling is dominated by the substrate affinity on hDAT, with negligible influence of L-type channel voltage sensitivity. In contrast, the maximal coupling-strength (defined as Ca(2+) signal change per unit hDAT current) is influenced by CaV voltage sensitivity, which is greater in CaV1.3- than in CaV1.2-expressing cells. Moreover, relative to DA, S(+)AMPH showed greater coupling-strength at concentrations that induced relatively small hDAT-mediated currents. Therefore S(+)AMPH is not only more potent than DA at inducing hDAT-mediated L-type Ca(2+) channel currents but is a better depolarizing agent since it produces tighter electrical coupling between hDAT-mediated depolarization and L-type Ca(2+) channel activation.
Keywords: Excitability; L-type Ca(2+) channels; MDMA; Monoamine transporters; Neurotransmitter transport; Serotonin; Stimulants.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
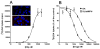




References
-
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12. - PubMed
-
- Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998;3:386–96. - PubMed
-
- Mignot E. Perspectives in narcolepsy research and therapy. Curr Opin Pulm Med. 1996;2:482–7. - PubMed
-
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. - PubMed
-
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

