Temperature-Dependent Galleria mellonella Mortality as a Result of Yersinia entomophaga Infection
- PMID: 26162867
- PMCID: PMC4542262
- DOI: 10.1128/AEM.00790-15
Temperature-Dependent Galleria mellonella Mortality as a Result of Yersinia entomophaga Infection
Abstract
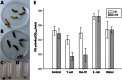
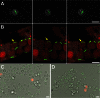
The bacterium Yersinia entomophaga is pathogenic to a range of insect species, with death typically occurring within 2 to 5 days of ingestion. Per os challenge of larvae of the greater wax moth (Galleria mellonella) confirmed that Y. entomophaga was virulent when fed to larvae held at 25°C but was avirulent when fed to larvae maintained at 37°C. At 25°C, a dose of ~4 × 10(7) CFU per larva of a Y. entomophaga toxin complex (Yen-TC) deletion derivative, the Y. entomophaga ΔTC variant, resulted in 27% mortality. This low level of activity was restored to near-wild-type levels by augmentation of the diet with a sublethal dose of purified Yen-TC. Intrahemocoelic injection of ~3 Y. entomophaga or Y. entomophaga ΔTC cells per larva gave a 4-day median lethal dose, with similar levels of mortality observed at both 25 and 37°C. Following intrahemocoelic injection of a Yen-TC YenA1 green fluorescent protein fusion strain into larvae maintained at 25°C, the bacteria did not fluoresce until the population density reached 2 × 10(7) CFU ml(-1) of hemolymph. The observed cells also took an irregular form. When the larvae were maintained at 37°C, the cells were small and the observed fluorescence was sporadic and weak, being more consistent at a population density of ~3 × 10(9) CFU ml(-1) of hemolymph. These findings provide further understanding of the pathobiology of Y. entomophaga in insects, showing that the bacterium gains direct access to the hemocoelic cavity, from where it rapidly multiplies to cause disease.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
The main virulence determinant of Yersinia entomophaga MH96 is a broad-host-range toxin complex active against insects.J Bacteriol. 2011 Apr;193(8):1966-80. doi: 10.1128/JB.01044-10. Epub 2011 Jan 28. J Bacteriol. 2011. PMID: 21278295 Free PMC article.
-
Assessment of toxicity and persistence of Yersinia entomophaga and its Yen-Tc associated toxin.Pest Manag Sci. 2020 Dec;76(12):4301-4310. doi: 10.1002/ps.5997. Epub 2020 Aug 18. Pest Manag Sci. 2020. PMID: 32648630
-
Assessment of Yersinia entomophaga as a control agent of the diamondback moth Plutella xylostella.J Invertebr Pathol. 2019 Mar;162:19-25. doi: 10.1016/j.jip.2019.02.002. Epub 2019 Feb 5. J Invertebr Pathol. 2019. PMID: 30735764
-
Pathology of Yersinia entomophaga MH96 towards Costelytra zealandica (Coleoptera; Scarabaeidae) larvae.J Invertebr Pathol. 2014 Jan;115:102-7. doi: 10.1016/j.jip.2013.11.004. Epub 2013 Nov 27. J Invertebr Pathol. 2014. PMID: 24291403
-
Temperature-dependent regulatory mechanism of larval development of the wax moth (Galleria mellonella).Acta Biochim Pol. 2000;47(1):215-21. Acta Biochim Pol. 2000. PMID: 10961696 Review.
Cited by
-
Biogenic Aspergillus tubingensis silver nanoparticles' in vitro effects on human umbilical vein endothelial cells, normal human fibroblasts, HEPG2, and Galleria mellonella.Toxicol Res (Camb). 2019 Oct 10;8(6):789-801. doi: 10.1039/c9tx00091g. eCollection 2019 Nov 1. Toxicol Res (Camb). 2019. PMID: 32206300 Free PMC article.
-
Infected insect gut reveals differentially expressed proteins for cellular redox, metal resistance and secretion system in Yersinia enterocolitica-Helicoverpa armigera pathogenic model.Biotechnol Lett. 2021 Sep;43(9):1845-1867. doi: 10.1007/s10529-021-03157-3. Epub 2021 Jun 24. Biotechnol Lett. 2021. PMID: 34165641
-
Toxigenic Properties of Yersinia enterocolitica Biotype 1A.Toxins (Basel). 2022 Feb 5;14(2):118. doi: 10.3390/toxins14020118. Toxins (Basel). 2022. PMID: 35202145 Free PMC article. Review.
-
Insecticidal Toxicity of Yersinia frederiksenii Involves the Novel Enterotoxin YacT.Front Cell Infect Microbiol. 2018 Nov 14;8:392. doi: 10.3389/fcimb.2018.00392. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30488025 Free PMC article.
-
Environmental interactions are regulated by temperature in Burkholderia seminalis TC3.4.2R3.Sci Rep. 2019 Apr 2;9(1):5486. doi: 10.1038/s41598-019-41778-x. Sci Rep. 2019. PMID: 30940839 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

