p53 suppresses hyper-recombination by modulating BRCA1 function
- PMID: 26162908
- PMCID: PMC4960982
- DOI: 10.1016/j.dnarep.2015.06.005
p53 suppresses hyper-recombination by modulating BRCA1 function
Abstract
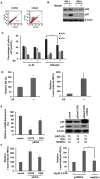
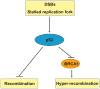
Both p53 and BRCA1 are tumor suppressors and are involved in a number of cellular processes including cell cycle arrest, apoptosis, transcriptional regulation, and DNA damage repair. Some studies have suggested that the association of BRCA1 and p53 is required for transcriptional regulation of genes involved in cell replication and DNA repair pathways. However, the relationship between the two proteins in molecular mechanisms of DNA repair is still not clear. Therefore, we sought to determine whether there is a functional link between p53 and BRCA1 in DNA repair. Firstly, using a plasmid recombination substrate, pDR-GFP, integrated into the genome of breast cancer cell line MCF7, we have demonstrated that p53 suppressed Rad51-mediated hyper-recombinational repair by two independent cell models of HPV-E6 induced p53 inactivation and p53 knockdown assay. Our study further indicated that p53 mediated homologous recombination (HR) through inhibiting BRCA1 over-function via mechanism of transcription regulation in response to DNA repair. Since it was found p53 and BRCA1 existed in a protein complex, indicating both proteins may be associated at post-transcriptional level. Moreover, defective p53-induced hyper-recombination was associated with cell radioresistance and chromosomal stability, strongly supporting the involvement of p53 in the inhibition of hyper-recombination, which led to genetic stability and cellular function in response to DNA damage. In addition, it was found that p53 loss rescued BRCA1 deficiency via recovering HR and chromosomal stability, suggesting that p53 is also involved in the HR-inhibition independently of BRCA1. Thus, our data indicated that p53 was involved in inhibiting recombination by both BRCA1-dependent and -independent mechanisms, and there is a functional link between p53-suppression and BRCA1-promotion in regulation of HR activity at transcription level and possible post-transcription level.
Keywords: BRCA1; Chromosomal stability; Homologous recombination; Hyper-recombination; p53.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures


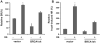


Similar articles
-
Homologous Recombination Repair Factors Rad51 and BRCA1 Are Necessary for Productive Replication of Human Papillomavirus 31.J Virol. 2015 Dec 23;90(5):2639-52. doi: 10.1128/JVI.02495-15. J Virol. 2015. PMID: 26699641 Free PMC article.
-
BRCA1 regulates RAD51 function in response to DNA damage and suppresses spontaneous sister chromatid replication slippage: implications for sister chromatid cohesion, genome stability, and carcinogenesis.Cancer Res. 2005 Dec 15;65(24):11384-91. doi: 10.1158/0008-5472.CAN-05-2156. Cancer Res. 2005. PMID: 16357146
-
BRCA1 interacts with dominant negative SWI/SNF enzymes without affecting homologous recombination or radiation-induced gene activation of p21 or Mdm2.J Cell Biochem. 2004 Apr 1;91(5):987-98. doi: 10.1002/jcb.20003. J Cell Biochem. 2004. PMID: 15034933
-
p53's double life: transactivation-independent repression of homologous recombination.Trends Genet. 2004 Jun;20(6):235-43. doi: 10.1016/j.tig.2004.04.003. Trends Genet. 2004. PMID: 15145576 Review.
-
Homologous recombination and cell cycle checkpoints: Rad51 in tumour progression and therapy resistance.Toxicology. 2003 Nov 15;193(1-2):91-109. doi: 10.1016/s0300-483x(03)00291-9. Toxicology. 2003. PMID: 14599770 Review.
Cited by
-
Prediction of lung adenocarcinoma prognosis and clinical treatment efficacy by telomere-associated gene risk model.Discov Oncol. 2025 Jun 14;16(1):1102. doi: 10.1007/s12672-025-02977-3. Discov Oncol. 2025. PMID: 40515885 Free PMC article.
-
Alterations in the p53 isoform ratio govern breast cancer cell fate in response to DNA damage.Cell Death Dis. 2022 Oct 28;13(10):907. doi: 10.1038/s41419-022-05349-9. Cell Death Dis. 2022. PMID: 36307393 Free PMC article.
-
Cell-type-specific epigenomic variations associated with BRCA1 mutation in pre-cancer human breast tissues.NAR Genom Bioinform. 2022 Feb 2;4(1):lqac006. doi: 10.1093/nargab/lqac006. eCollection 2022 Mar. NAR Genom Bioinform. 2022. PMID: 35118379 Free PMC article.
-
PRKCSH enhances colorectal cancer radioresistance via IRE1α/XBP1s-mediated DNA repair.Cell Death Dis. 2025 Apr 6;16(1):258. doi: 10.1038/s41419-025-07582-4. Cell Death Dis. 2025. PMID: 40189587 Free PMC article.
-
A linear programming computational framework integrates phosphor-proteomics and prior knowledge to predict drug efficacy.BMC Syst Biol. 2017 Dec 21;11(Suppl 7):127. doi: 10.1186/s12918-017-0501-6. BMC Syst Biol. 2017. PMID: 29322918 Free PMC article.
References
-
- Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. - PubMed
-
- Traverso G, Bettegowda C, Kraus J, Speicher MR, Kinzler KW, Vogelstein B, Lengauer C. Hyper-recombination and genetic instability in BLM-deficient epithelial cells. Cancer Res. 2003;63:8578–8581. - PubMed
-
- Huang Y, Boynton RF, Blount PL, Silverstein RJ, Yin J, Tong Y, McDaniel TK, Newkirk C, Resau JH, Sridhara R. Loss of heterozygosity involves multiple tumor suppressor genes in human esophageal cancers. Cancer Res. 1992;52:6525–6530. - PubMed
-
- Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6:44–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

