Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: a randomised controlled trial
- PMID: 26163028
- PMCID: PMC4499724
- DOI: 10.1136/bmjopen-2014-006564
Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: a randomised controlled trial
Abstract
Objectives: To assess the effect of the probiotic VSL#3 in prevention of neonatal sepsis in low birthweight (LBW) infants.
Design: Randomised, double-blind, placebo-controlled trial.
Setting: Community setting in rural India.
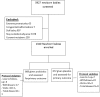
Participants: LBW infants aged 3-7 days.
Interventions: Infants were randomised to receive probiotic (VSL#3, 10 billion colony-forming units (cfu)) or placebo for 30 days, and were followed up for 2 months.
Main outcome measure: Possible serious bacterial infection (PSBI) as per the Integrated Management of Neonatal Childhood Illnesses algorithm, as diagnosed by fieldworkers/physicians.
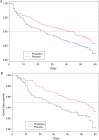
Results: 668 infants were randomised to VSL#3 and 672 to placebo. By intention-to-treat analysis, the risk of PSBI among infants in the overall population of LBW infants was not statistically significant (RR 0.79 (95% CI 0.56 to 1.03)). Probiotics reduced median days of hospitalisation (6 days vs 3 days in probiotics) (p=0.018) but not the risk of hospitalisation (RR 0.66 (95% CI 0.42 to 1.04). The onset of PSBI in 10% of infants occurred on the 40th day in the probiotics arm versus the 25th day in the control arm (p=0.063).
Conclusions: Daily supplementation of LBW infants with probiotics VSL#3 (10 billion cfu) for 30 days led to a non-significant 21% reduction in risk of neonatal sepsis. A larger study with sufficient power and a more specific primary end point is warranted to confirm the preventive effect of VSL#3 on neonatal sepsis in LBW infants.
Trial registration number: The study is registered at the Clinical Trial Registry of India (CTRI/2008/091/000049).
Keywords: NEONATOLOGY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Comment in
-
Probiotics for prevention of suspected sepsis in low birthweight infants.Acta Paediatr. 2017 Apr;106(4):681. doi: 10.1111/apa.13704. Epub 2017 Jan 31. Acta Paediatr. 2017. PMID: 28145021 No abstract available.
References
-
- Guidelines for the evaluation of probiotics in food: report of a joint FAO/WHO Working Group. London, Ontario, Canada: Food and Agriculture Organization of the United Nations and World Health Organization, 2002.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical