Recent insights into structures and functions of C-type lectins in the immune system
- PMID: 26163333
- PMCID: PMC4681411
- DOI: 10.1016/j.sbi.2015.06.003
Recent insights into structures and functions of C-type lectins in the immune system
Abstract
The majority of the C-type lectin-like domains in the human genome likely to bind sugars have been investigated structurally, although novel mechanisms of sugar binding are still being discovered. In the immune system, adhesion and endocytic receptors that bind endogenous mammalian glycans are often conserved, while pathogen-binding C-type lectins on cells of the innate immune system are more divergent. Lack of orthology between some human and mouse receptors, as well as overlapping specificities of many receptors and formation of receptor hetero-oligomers, can make it difficult to define the roles of individual receptors. There is good evidence that C-type lectins initiate signalling pathways in several different ways, but this function remains the least well understood from a mechanistic perspective.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
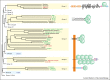

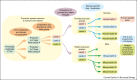

References
-
- Sharon N., Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. - PubMed
-
- Weis W.I., Taylor M.E., Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. - PubMed
-
- Lühn K., Wild M.K. Human deficiencies of fucosylation and sialylation affecting selectin ligands. Semin Immunopathol. 2012;34:383–399. - PubMed
-
- Keizer M.P., Wouters D., Schlapbach L.J., Kuijpers T.W. Restoration of MBL-deficiency: redefining the safety, efficacy and viability of MBL-substitution therapy. Mol Immunol. 2014;61:174–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

