NRG Oncology Radiation Therapy Oncology Group 0822: A Phase 2 Study of Preoperative Chemoradiation Therapy Using Intensity Modulated Radiation Therapy in Combination With Capecitabine and Oxaliplatin for Patients With Locally Advanced Rectal Cancer
- PMID: 26163334
- PMCID: PMC4540628
- DOI: 10.1016/j.ijrobp.2015.05.005
NRG Oncology Radiation Therapy Oncology Group 0822: A Phase 2 Study of Preoperative Chemoradiation Therapy Using Intensity Modulated Radiation Therapy in Combination With Capecitabine and Oxaliplatin for Patients With Locally Advanced Rectal Cancer
Abstract
Purpose: To evaluate the rate of gastrointestinal (GI) toxicity of neoadjuvant chemoradiation with capecitabine, oxaliplatin, and intensity modulated radiation therapy (IMRT) in cT3-4 rectal cancer.
Methods and materials: Patients with localized, nonmetastatic T3 or T4 rectal cancer <12 cm from the anal verge were enrolled in a prospective, multi-institutional, single-arm study of preoperative chemoradiation. Patients received 45 Gy with IMRT in 25 fractions, followed by a 3-dimensional conformal boost of 5.4 Gy in 3 fractions with concurrent capecitabine/oxaliplatin (CAPOX). Surgery was performed 4 to 8 weeks after the completion of therapy. Patients were recommended to receive FOLFOX chemotherapy after surgery. The primary endpoint of the study was acute grade 2 to 5 GI toxicity. Seventy-one patients provided 80% probability to detect at least a 12% reduction in the specified GI toxicity with the treatment of CAPOX and IMRT, at a significance level of .10 (1-sided).
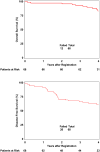
Results: Seventy-nine patients were accrued, of whom 68 were evaluable. Sixty-one patients (89.7%) had cT3 disease, and 37 (54.4%) had cN (+) disease. Postoperative chemotherapy was given to 42 of 68 patients. Fifty-eight patients had target contours drawn per protocol, 5 patients with acceptable variation, and 5 patients with unacceptable variations. Thirty-five patients (51.5%) experienced grade ≥ 2 GI toxicity, 12 patients (17.6%) experienced grade 3 or 4 diarrhea, and pCR was achieved in 10 patients (14.7%). With a median follow-up time of 3.98 years, the 4-year rate of locoregional failure was 7.4% (95% confidence interval [CI]: 1.0%-13.7%). The 4-year rates of OS and DFS were 82.9% (95% CI: 70.1%-90.6%) and 60.6% (95% CI: 47.5%-71.4%), respectively.
Conclusion: The use of IMRT in neoadjuvant chemoradiation for rectal cancer did not reduce the rate of GI toxicity.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest- None
Figures
Similar articles
-
Four-week neoadjuvant intensity-modulated radiation therapy with concurrent capecitabine and oxaliplatin in locally advanced rectal cancer patients: a validation phase II trial.Int J Radiat Oncol Biol Phys. 2012 Jun 1;83(2):587-93. doi: 10.1016/j.ijrobp.2011.06.2008. Epub 2011 Nov 11. Int J Radiat Oncol Biol Phys. 2012. PMID: 22079731 Clinical Trial.
-
Radiation Therapy Oncology Group 0247: a randomized Phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer.Int J Radiat Oncol Biol Phys. 2012 Mar 15;82(4):1367-75. doi: 10.1016/j.ijrobp.2011.05.027. Epub 2011 Jul 19. Int J Radiat Oncol Biol Phys. 2012. PMID: 21775070 Free PMC article. Clinical Trial.
-
A phase II trial of neoadjuvant IMRT-based chemoradiotherapy followed by one cycle of capecitabine for stage II/III rectal adenocarcinoma.Radiat Oncol. 2013 May 29;8:130. doi: 10.1186/1748-717X-8-130. Radiat Oncol. 2013. PMID: 23718210 Free PMC article. Clinical Trial.
-
Efficacy endpoints of radiation therapy group protocol 0247: a randomized, phase 2 study of neoadjuvant radiation therapy plus concurrent capecitabine and irinotecan or capecitabine and oxaliplatin for patients with locally advanced rectal cancer.Int J Radiat Oncol Biol Phys. 2015 Jan 1;91(1):116-23. doi: 10.1016/j.ijrobp.2014.09.031. Epub 2014 Nov 5. Int J Radiat Oncol Biol Phys. 2015. PMID: 25446610 Free PMC article. Clinical Trial.
-
Role of Oxaliplatin in the Neoadjuvant Concurrent Chemoradiotherapy in Locally Advanced Rectal Cancer: a Review of Evidence.Clin Med Insights Oncol. 2024 Mar 19;18:11795549241236409. doi: 10.1177/11795549241236409. eCollection 2024. Clin Med Insights Oncol. 2024. PMID: 38510317 Free PMC article. Review.
Cited by
-
Neoadjuvant PET and MRI-based intensity modulated radiotherapy leads to less toxicity and improved pathologic response rates in locally advanced rectal cancer.J Gastrointest Oncol. 2018 Aug;9(4):641-649. doi: 10.21037/jgo.2018.03.10. J Gastrointest Oncol. 2018. PMID: 30151260 Free PMC article.
-
Dose-time fractionation schedules of preoperative radiotherapy and timing to surgery for rectal cancer.Ther Adv Med Oncol. 2020 Feb 29;12:1758835920907537. doi: 10.1177/1758835920907537. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 32165928 Free PMC article. Review.
-
Adaptive radiation dose escalation in rectal adenocarcinoma: a review.J Gastrointest Oncol. 2017 Oct;8(5):902-914. doi: 10.21037/jgo.2017.07.06. J Gastrointest Oncol. 2017. PMID: 29184696 Free PMC article. Review.
-
Gender-Affirming Surgery and Cancer: Considerations for Radiation Oncologists for Pelvic Radiation in Transfeminine Patients.Int J Radiat Oncol Biol Phys. 2023 Oct 1;117(2):301-311. doi: 10.1016/j.ijrobp.2023.05.028. Epub 2023 May 24. Int J Radiat Oncol Biol Phys. 2023. PMID: 37230432 Free PMC article. Review.
-
Replacing manual planning with automatic iterative planning for locally advanced rectal cancer VMAT treatment.J Appl Clin Med Phys. 2025 Jan;26(1):e14552. doi: 10.1002/acm2.14552. Epub 2024 Oct 15. J Appl Clin Med Phys. 2025. PMID: 39406255 Free PMC article.
References
-
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80. - PubMed
-
- Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvannt chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405 PRODIGE 2. J Clin Oncol. 2010;28:1638–44. - PubMed
-
- Wong SJ, Winter K, Meropol NJ, et al. Radiation Therapy Oncology Group 0247: a randomized Phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:1367–75. - PMC - PubMed
-
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources