Activation of the nuclear factor-κB pathway during postnatal lung inflammation preserves alveolarization by suppressing macrophage inflammatory protein-2
- PMID: 26163511
- PMCID: PMC4572419
- DOI: 10.1152/ajplung.00029.2015
Activation of the nuclear factor-κB pathway during postnatal lung inflammation preserves alveolarization by suppressing macrophage inflammatory protein-2
Abstract
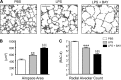
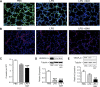
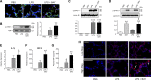
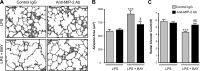
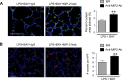
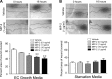
A significant portion of lung development is completed postnatally during alveolarization, rendering the immature lung vulnerable to inflammatory stimuli that can disrupt lung structure and function. Although the NF-κB pathway has well-recognized pro-inflammatory functions, novel anti-inflammatory and developmental roles for NF-κB have recently been described. Thus, to determine how NF-κB modulates alveolarization during inflammation, we exposed postnatal day 6 mice to vehicle (PBS), systemic lipopolysaccharide (LPS), or the combination of LPS and the global NF-κB pathway inhibitor BAY 11-7082 (LPS + BAY). LPS impaired alveolarization, decreased lung cell proliferation, and reduced epithelial growth factor expression. BAY exaggerated these detrimental effects of LPS, further suppressing proliferation and disrupting pulmonary angiogenesis, an essential component of alveolarization. The more severe pathology induced by LPS + BAY was associated with marked increases in lung and plasma levels of macrophage inflammatory protein-2 (MIP-2). Experiments using primary neonatal pulmonary endothelial cells (PEC) demonstrated that MIP-2 directly impaired neonatal PEC migration in vitro; and neutralization of MIP-2 in vivo preserved lung cell proliferation and pulmonary angiogenesis and prevented the more severe alveolar disruption induced by the combined treatment of LPS + BAY. Taken together, these studies demonstrate a key anti-inflammatory function of the NF-κB pathway in the early alveolar lung that functions to mitigate the detrimental effects of inflammation on pulmonary angiogenesis and alveolarization. Furthermore, these data suggest that neutralization of MIP-2 may represent a novel therapeutic target that could be beneficial in preserving lung growth in premature infants exposed to inflammatory stress.
Keywords: angiogenesis; bronchopulmonary dysplasia; endothelial migration; proliferation.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization.Am J Physiol Lung Cell Mol Physiol. 2012 May 15;302(10):L1023-36. doi: 10.1152/ajplung.00230.2011. Epub 2012 Feb 24. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22367785 Free PMC article.
-
Transforming Growth Factor-induced Protein Promotes NF-κB-mediated Angiogenesis during Postnatal Lung Development.Am J Respir Cell Mol Biol. 2021 Mar;64(3):318-330. doi: 10.1165/rcmb.2020-0153OC. Am J Respir Cell Mol Biol. 2021. PMID: 33264084 Free PMC article.
-
Endothelial-specific loss of IKKβ disrupts pulmonary endothelial angiogenesis and impairs postnatal lung growth.Am J Physiol Lung Cell Mol Physiol. 2023 Sep 1;325(3):L299-L313. doi: 10.1152/ajplung.00034.2023. Epub 2023 Jun 13. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37310763 Free PMC article.
-
Effect of Intranasal Instillation of Lipopolysaccharide on Lung Development and Its Related Mechanism in Newborn Mice.J Interferon Cytokine Res. 2019 Nov;39(11):684-693. doi: 10.1089/jir.2019.0006. Epub 2019 Jul 3. J Interferon Cytokine Res. 2019. PMID: 31268385 Free PMC article.
-
Nuclear factor-kappa-B signaling in lung development and disease: one pathway, numerous functions.Birth Defects Res A Clin Mol Teratol. 2014 Mar;100(3):202-16. doi: 10.1002/bdra.23233. Epub 2014 Mar 17. Birth Defects Res A Clin Mol Teratol. 2014. PMID: 24639404 Free PMC article. Review.
Cited by
-
TRAIL protects the immature lung from hyperoxic injury.Cell Death Dis. 2022 Jul 15;13(7):614. doi: 10.1038/s41419-022-05072-5. Cell Death Dis. 2022. PMID: 35840556 Free PMC article.
-
The Potentials and Caveats of Mesenchymal Stromal Cell-Based Therapies in the Preterm Infant.Stem Cells Int. 2018 Apr 8;2018:9652897. doi: 10.1155/2018/9652897. eCollection 2018. Stem Cells Int. 2018. PMID: 29765429 Free PMC article. Review.
-
NF-κB in Oxidative Stress.Curr Opin Toxicol. 2018 Feb;7:81-86. doi: 10.1016/j.cotox.2017.11.002. Epub 2017 Nov 7. Curr Opin Toxicol. 2018. PMID: 29862377 Free PMC article.
-
Lung development and immune status under chronic LPS exposure in rat pups with and without CD26/DPP4 deficiency.Cell Tissue Res. 2021 Dec;386(3):617-636. doi: 10.1007/s00441-021-03522-8. Epub 2021 Oct 4. Cell Tissue Res. 2021. PMID: 34606000 Free PMC article.
-
Intrauterine growth restriction decreases NF-κB signaling in fetal pulmonary artery endothelial cells of fetal sheep.Am J Physiol Lung Cell Mol Physiol. 2018 Sep 1;315(3):L348-L359. doi: 10.1152/ajplung.00052.2018. Epub 2018 May 3. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29722560 Free PMC article.
References
-
- Aoki K, Ishida Y, Kikuta N, Kawai H, Kuroiwa M, Sato H. Role of CXC chemokines in the enhancement of LPS-induced neutrophil accumulation in the lung of mice by dexamethasone. Biochem Biophys Res Commun 294: 1101–1108, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

