Combined Liver X Receptor/Peroxisome Proliferator-activated Receptor γ Agonist Treatment Reduces Amyloid β Levels and Improves Behavior in Amyloid Precursor Protein/Presenilin 1 Mice
- PMID: 26163517
- PMCID: PMC4571883
- DOI: 10.1074/jbc.M115.652008
Combined Liver X Receptor/Peroxisome Proliferator-activated Receptor γ Agonist Treatment Reduces Amyloid β Levels and Improves Behavior in Amyloid Precursor Protein/Presenilin 1 Mice
Abstract
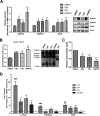
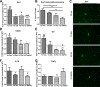
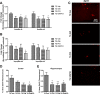
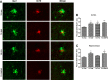
Alzheimer disease (AD) is characterized by the extracellular accumulation of amyloid β (Aβ), which is accompanied by a robust inflammatory response in the brain. Both of these pathogenic processes are regulated by nuclear receptors, including the liver X receptors (LXRs) and peroxisome-proliferator receptor γ (PPARγ). Agonists of LXRs have been demonstrated previously to reduce Aβ levels and improve cognitive deficits in AD mouse models by inducing the transcription and lipidation of apolipoprotein E (apoE). Agonists targeting PPARγ reduce the microglial expression of proinflammatory genes and have also been shown to modulate apoE expression. Here we investigate whether a combination therapy with both LXR and PPARγ agonists results in increased benefits in an AD mouse model. We found that the LXR agonist GW3965 and the PPARγ agonist pioglitazone were individually able to increase the levels of apoE and related genes, decrease the expression of proinflammatory genes, and facilitate Aβ decreases in the hippocampus. Combined treatment with both agonists provoked a further increase in the expression of apoE and a decrease in the soluble and deposited forms of Aβ. The decrease in plaques was associated with increased colocalization between microglia and plaques. In addition, the PPARγ agonist in the combined treatment paradigm was able to counteract the elevation in plasma triglycerides that is a side effect of LXR agonist treatment. These results suggest that combined LXR/PPARγ agonist treatment merits further investigation for the treatment of AD.
Keywords: Alzheimer disease; amyloid β (Aβ); apolipoprotein E (apoE); liver X receptor (LXR); microglia; peroxisome proliferator-activated receptor (PPAR).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer's disease.J Neurosci. 2012 Jul 25;32(30):10117-28. doi: 10.1523/JNEUROSCI.5268-11.2012. J Neurosci. 2012. PMID: 22836247 Free PMC article.
-
PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice.J Neurosci. 2012 Nov 28;32(48):17321-31. doi: 10.1523/JNEUROSCI.1569-12.2012. J Neurosci. 2012. PMID: 23197723 Free PMC article.
-
ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice.J Biol Chem. 2010 Oct 29;285(44):34144-54. doi: 10.1074/jbc.M110.108100. Epub 2010 Aug 25. J Biol Chem. 2010. PMID: 20739291 Free PMC article.
-
Greasing the wheels of Abeta clearance in Alzheimer's disease: the role of lipids and apolipoprotein E.Biofactors. 2009 May-Jun;35(3):239-48. doi: 10.1002/biof.37. Biofactors. 2009. PMID: 19472365 Review.
-
Current Progress on Peroxisome Proliferator-activated Receptor Gamma Agonist as an Emerging Therapeutic Approach for the Treatment of Alzheimer's Disease: An Update.Curr Neuropharmacol. 2019;17(3):232-246. doi: 10.2174/1570159X16666180828100002. Curr Neuropharmacol. 2019. PMID: 30152284 Free PMC article. Review.
Cited by
-
Combination treatment with leptin and pioglitazone in a mouse model of Alzheimer's disease.Alzheimers Dement (N Y). 2016 Dec 20;3(1):92-106. doi: 10.1016/j.trci.2016.11.002. eCollection 2017 Jan. Alzheimers Dement (N Y). 2016. PMID: 29067321 Free PMC article.
-
Is Alzheimer's Disease a Liver Disease of the Brain?J Alzheimers Dis. 2020;75(1):1-14. doi: 10.3233/JAD-190848. J Alzheimers Dis. 2020. PMID: 32250293 Free PMC article. Review.
-
Association between genetically proxied PPARG activation and early onset Alzheimer's disease: A drug target Mendelian randomization study.J Alzheimers Dis Rep. 2025 Jul 21;9:25424823251359579. doi: 10.1177/25424823251359579. eCollection 2025 Jan-Dec. J Alzheimers Dis Rep. 2025. PMID: 40697193 Free PMC article.
-
Role of Oxysterols in the Activation of the NLRP3 Inflammasome as a Potential Pharmacological Approach in Alzheimer's Disease.Curr Neuropharmacol. 2023;21(2):202-212. doi: 10.2174/1570159X20666220327215245. Curr Neuropharmacol. 2023. PMID: 35339182 Free PMC article. Review.
-
Diabetes: Risk factor and translational therapeutic implications for Alzheimer's disease.Eur J Neurosci. 2022 Nov;56(9):5727-5757. doi: 10.1111/ejn.15619. Epub 2022 Feb 23. Eur J Neurosci. 2022. PMID: 35128745 Free PMC article.
References
-
- Castellano J. M., Kim J., Stewart F. R., Jiang H., DeMattos R. B., Patterson B. W., Fagan A. M., Morris J. C., Mawuenyega K. G., Cruchaga C., Goate A. M., Bales K. R., Paul S. M., Bateman R. J., Holtzman D. M. (2011) Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3, 89ra57 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

