Aquaporins Contribute to ABA-Triggered Stomatal Closure through OST1-Mediated Phosphorylation
- PMID: 26163575
- PMCID: PMC4531361
- DOI: 10.1105/tpc.15.00421
Aquaporins Contribute to ABA-Triggered Stomatal Closure through OST1-Mediated Phosphorylation
Abstract
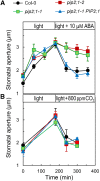
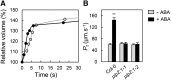
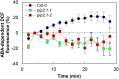
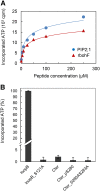
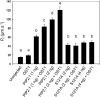
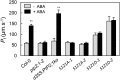
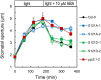
Stomatal movements in response to environmental stimuli critically control the plant water status. Although these movements are governed by osmotically driven changes in guard cell volume, the role of membrane water channels (aquaporins) has remained hypothetical. Assays in epidermal peels showed that knockout Arabidopsis thaliana plants lacking the Plasma membrane Intrinsic Protein 2;1 (PIP2;1) aquaporin have a defect in stomatal closure, specifically in response to abscisic acid (ABA). ABA induced a 2-fold increase in osmotic water permeability (Pf) of guard cell protoplasts and an accumulation of reactive oxygen species in guard cells, which were both abrogated in pip2;1 plants. Open stomata 1 (OST1)/Snf1-related protein kinase 2.6 (SnRK2.6), a protein kinase involved in guard cell ABA signaling, was able to phosphorylate a cytosolic PIP2;1 peptide at Ser-121. OST1 enhanced PIP2;1 water transport activity when coexpressed in Xenopus laevis oocytes. Upon expression in pip2;1 plants, a phosphomimetic form (Ser121Asp) but not a phosphodeficient form (Ser121Ala) of PIP2;1 constitutively enhanced the Pf of guard cell protoplasts while suppressing its ABA-dependent activation and was able to restore ABA-dependent stomatal closure in pip2;1. This work supports a model whereby ABA-triggered stomatal closure requires an increase in guard cell permeability to water and possibly hydrogen peroxide, through OST1-dependent phosphorylation of PIP2;1 at Ser-121.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures
Similar articles
-
Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure.Proc Natl Acad Sci U S A. 2017 Aug 22;114(34):9200-9205. doi: 10.1073/pnas.1704754114. Epub 2017 Aug 7. Proc Natl Acad Sci U S A. 2017. PMID: 28784763 Free PMC article.
-
A link between magnesium-chelatase H subunit and sucrose nonfermenting 1 (SNF1)-related protein kinase SnRK2.6/OST1 in Arabidopsis guard cell signalling in response to abscisic acid.J Exp Bot. 2015 Oct;66(20):6355-69. doi: 10.1093/jxb/erv341. Epub 2015 Jul 13. J Exp Bot. 2015. PMID: 26175350 Free PMC article.
-
Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells.New Phytol. 2013 Dec;200(4):1049-63. doi: 10.1111/nph.12469. Epub 2013 Sep 3. New Phytol. 2013. PMID: 24033256
-
Protein phosphorylation in stomatal movement.Plant Signal Behav. 2014;9(11):e972845. doi: 10.4161/15592316.2014.972845. Plant Signal Behav. 2014. PMID: 25482764 Free PMC article. Review.
-
Nitric oxide, stomatal closure, and abiotic stress.J Exp Bot. 2008;59(2):165-76. doi: 10.1093/jxb/erm293. J Exp Bot. 2008. PMID: 18332225 Review.
Cited by
-
Stress-induced reactive oxygen species compartmentalization, perception and signalling.Nat Plants. 2021 Apr;7(4):403-412. doi: 10.1038/s41477-021-00887-0. Epub 2021 Apr 12. Nat Plants. 2021. PMID: 33846592 Free PMC article. Review.
-
A Cytoplasmic Receptor-like Kinase Contributes to Salinity Tolerance.Plants (Basel). 2020 Oct 17;9(10):1383. doi: 10.3390/plants9101383. Plants (Basel). 2020. PMID: 33080797 Free PMC article.
-
The site of water stress governs the pattern of ABA synthesis and transport in peanut.Sci Rep. 2016 Oct 3;6:32143. doi: 10.1038/srep32143. Sci Rep. 2016. PMID: 27694957 Free PMC article.
-
Hormonal and environmental signaling pathways target membrane water transport.Plant Physiol. 2021 Dec 4;187(4):2056-2070. doi: 10.1093/plphys/kiab373. Plant Physiol. 2021. PMID: 35235672 Free PMC article. Review.
-
Protein Phosphorylation Response to Abiotic Stress in Plants.Methods Mol Biol. 2021;2358:17-43. doi: 10.1007/978-1-0716-1625-3_2. Methods Mol Biol. 2021. PMID: 34270044 Review.
References
-
- Ache P., Bauer H., Kollist H., Al-Rasheid K.A., Lautner S., Hartung W., Hedrich R. (2010). Stomatal action directly feeds back on leaf turgor: new insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J. 62: 1072–1082. - PubMed
-
- Brandt B., Brodsky D.E., Xue S., Negi J., Iba K., Kangasjärvi J., Ghassemian M., Stephan A.B., Hu H., Schroeder J.I. (2012). Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA 109: 10593–10598. - PMC - PubMed
-
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

