Dual Function of the IRF8 Transcription Factor in Autoimmune Uveitis: Loss of IRF8 in T Cells Exacerbates Uveitis, Whereas Irf8 Deletion in the Retina Confers Protection
- PMID: 26163590
- PMCID: PMC4530071
- DOI: 10.4049/jimmunol.1500653
Dual Function of the IRF8 Transcription Factor in Autoimmune Uveitis: Loss of IRF8 in T Cells Exacerbates Uveitis, Whereas Irf8 Deletion in the Retina Confers Protection
Abstract
IFN regulatory factor 8 (IRF8) is constitutively expressed in monocytes and B cells and plays a critical role in the functional maturation of microglia cells. It is induced in T cells following Ag stimulation, but its functions are less well understood. However, recent studies in mice with T cell-specific Irf8 disruption under direction of the Lck promoter (LCK-IRF8KO) suggest that IRF8 directs a silencing program for Th17 differentiation, and IL-17 production is markedly increased in IRF8-deficient T cells. Paradoxically, loss of IRF8 in T cells has no effect on the development or severity of experimental autoimmune encephalomyelitis (EAE), although exacerbating colitis in a mouse colitis model. In contrast, mice with a macrophage/microglia-specific Irf8 disruption are resistant to EAE, further confounding our understanding of the roles of IRF8 in host immunity and autoimmunity. To clarify the role of IRF8 in autoimmune diseases, we have generated two mouse strains with targeted deletion of Irf8 in retinal cells, including microglial cells and a third mouse strain with targeted Irf8 deletion in T cells under direction of the nonpromiscuous, CD4 promoter (CD4-IRF8KO). In contrast to the report that IRF8 deletion in T cells has no effect on EAE, experimental autoimmune uveitis is exacerbated in CD4-IRF8KO mice and disease enhancement correlates with significant expansion of Th17 cells and a reduction in T regulatory cells. In contrast to CD4-IRF8KO mice, Irf8 deletion in retinal cells confers protection from uveitis, underscoring divergent and tissue-specific roles of IRF8 in host immunity. These results raise a cautionary note in the context of therapeutic targeting of IRF8.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



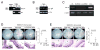
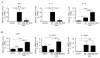

Similar articles
-
Deletion of Irf4 in T Cells Suppressed Autoimmune Uveitis and Dysregulated Transcriptional Programs Linked to CD4+ T Cell Differentiation and Metabolism.Int J Mol Sci. 2021 Mar 9;22(5):2775. doi: 10.3390/ijms22052775. Int J Mol Sci. 2021. PMID: 33803441 Free PMC article.
-
Therapeutic targeting of STAT3 (signal transducers and activators of transcription 3) pathway inhibits experimental autoimmune uveitis.PLoS One. 2012;7(1):e29742. doi: 10.1371/journal.pone.0029742. Epub 2012 Jan 5. PLoS One. 2012. PMID: 22238646 Free PMC article.
-
Mincle Activation and the Syk/Card9 Signaling Axis Are Central to the Development of Autoimmune Disease of the Eye.J Immunol. 2016 Apr 1;196(7):3148-58. doi: 10.4049/jimmunol.1502355. Epub 2016 Feb 26. J Immunol. 2016. PMID: 26921309 Free PMC article.
-
Mouse Models of Experimental Autoimmune Uveitis: Comparative Analysis of Adjuvant-Induced vs Spontaneous Models of Uveitis.Curr Mol Med. 2015;15(6):550-7. doi: 10.2174/1566524015666150731100318. Curr Mol Med. 2015. PMID: 26238369 Free PMC article. Review.
-
The Role of αA-Crystallin in Experimental Autoimmune Uveitis.Curr Mol Med. 2015;15(6):558-64. doi: 10.2174/1566524015666150731101146. Curr Mol Med. 2015. PMID: 26238368 Review.
Cited by
-
High IRF8 expression correlates with CD8 T cell infiltration and is a predictive biomarker of therapy response in ER-negative breast cancer.Breast Cancer Res. 2021 Mar 25;23(1):40. doi: 10.1186/s13058-021-01418-7. Breast Cancer Res. 2021. PMID: 33766090 Free PMC article.
-
Hypermethylation of Interferon Regulatory Factor 8 (IRF8) Confers Risk to Vogt-Koyanagi-Harada Disease.Sci Rep. 2017 Apr 21;7(1):1007. doi: 10.1038/s41598-017-01249-7. Sci Rep. 2017. PMID: 28432342 Free PMC article.
-
Kinetic changes in microglia-related retinal transcripts in experimental autoimmune uveoretinitis (EAU) of B10.RIII mice.J Neuroinflammation. 2025 Feb 10;22(1):37. doi: 10.1186/s12974-025-03358-x. J Neuroinflammation. 2025. PMID: 39930455 Free PMC article.
-
Therapeutic Targeting of IRFs: Pathway-Dependence or Structure-Based?Front Immunol. 2018 Nov 20;9:2622. doi: 10.3389/fimmu.2018.02622. eCollection 2018. Front Immunol. 2018. PMID: 30515152 Free PMC article. Review.
-
IRF8: Mechanism of Action and Health Implications.Cells. 2022 Aug 24;11(17):2630. doi: 10.3390/cells11172630. Cells. 2022. PMID: 36078039 Free PMC article. Review.
References
-
- Lohoff M, Mak TW. Roles of interferon-regulatory factors in T-helper-cell differentiation. Nat Rev Immunol. 2005;5:125–135. - PubMed
-
- Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. - PubMed
-
- Nelson N, Kanno Y, Hong C, Contursi C, Fujita T, Fowlkes BJ, O’Connell E, Hu-Li J, Paul WE, Jankovic D, Sher AF, Coligan JE, Thornton A, Appella E, Yang Y, Ozato K. Expression of IFN regulatory factor family proteins in lymphocytes. Induction of Stat-1 and IFN consensus sequence binding protein expression by T cell activation. J Immunol. 1996;156:3711–3720. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

