Differential inhibition onto developing and mature granule cells generates high-frequency filters with variable gain
- PMID: 26163657
- PMCID: PMC4521582
- DOI: 10.7554/eLife.08764
Differential inhibition onto developing and mature granule cells generates high-frequency filters with variable gain
Abstract
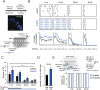
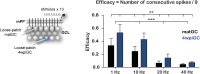
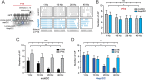
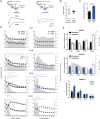
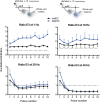
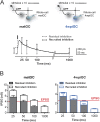
Adult hippocampal neurogenesis provides the dentate gyrus with heterogeneous populations of granule cells (GC) originated at different times. The contribution of these cells to information encoding is under current investigation. Here, we show that incoming spike trains activate different populations of GC determined by the stimulation frequency and GC age. Immature GC respond to a wider range of stimulus frequencies, whereas mature GC are less responsive at high frequencies. This difference is dictated by feedforward inhibition, which restricts mature GC activation. Yet, the stronger inhibition of mature GC results in a higher temporal fidelity compared to that of immature GC. Thus, hippocampal inputs activate two populations of neurons with variable frequency filters: immature cells, with wide-range responses, that are reliable transmitters of the incoming frequency, and mature neurons, with narrow frequency response, that are precise at informing the beginning of the stimulus, but with a sparse activity.
Keywords: adult neurogenesis; excitation-inhibition; hippocampus; mouse; neuroscience.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures





Similar articles
-
Unique processing during a period of high excitation/inhibition balance in adult-born neurons.Science. 2012 Mar 9;335(6073):1238-42. doi: 10.1126/science.1214956. Epub 2012 Jan 26. Science. 2012. PMID: 22282476 Free PMC article.
-
Low excitatory innervation balances high intrinsic excitability of immature dentate neurons.Nat Commun. 2016 Apr 20;7:11313. doi: 10.1038/ncomms11313. Nat Commun. 2016. PMID: 27095423 Free PMC article.
-
Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys.J Comp Neurol. 2004 Aug 23;476(3):205-18. doi: 10.1002/cne.20182. J Comp Neurol. 2004. PMID: 15269966
-
Dynamic role of adult-born dentate granule cells in memory processing.Curr Opin Neurobiol. 2015 Dec;35:21-6. doi: 10.1016/j.conb.2015.06.002. Epub 2015 Jun 19. Curr Opin Neurobiol. 2015. PMID: 26100379 Free PMC article. Review.
-
How to make a hippocampal dentate gyrus granule neuron.Development. 2014 Jun;141(12):2366-75. doi: 10.1242/dev.096776. Development. 2014. PMID: 24917496 Review.
Cited by
-
Multifaceted circuit functions of adult-born neurons.F1000Res. 2019 Nov 26;8:F1000 Faculty Rev-1998. doi: 10.12688/f1000research.20642.1. eCollection 2019. F1000Res. 2019. PMID: 31824650 Free PMC article. Review.
-
Deficits in Behavioral and Neuronal Pattern Separation in Temporal Lobe Epilepsy.J Neurosci. 2021 Nov 17;41(46):9669-9686. doi: 10.1523/JNEUROSCI.2439-20.2021. Epub 2021 Oct 7. J Neurosci. 2021. PMID: 34620720 Free PMC article.
-
Causal Evidence for the Role of Specific GABAergic Interneuron Types in Entorhinal Recruitment of Dentate Granule Cells.Sci Rep. 2016 Nov 10;6:36885. doi: 10.1038/srep36885. Sci Rep. 2016. PMID: 27830729 Free PMC article.
-
Pattern separation of spiketrains in hippocampal neurons.Sci Rep. 2019 Mar 27;9(1):5282. doi: 10.1038/s41598-019-41503-8. Sci Rep. 2019. PMID: 30918288 Free PMC article.
-
Massively augmented hippocampal dentate granule cell activation accompanies epilepsy development.Sci Rep. 2017 Feb 20;7:42090. doi: 10.1038/srep42090. Sci Rep. 2017. PMID: 28218241 Free PMC article.
References
-
- Andersen P, Bliss TV, Skrede KK. Lamellar organization of hippocampal pathways. Experimental Brain Research. 1971;13:222–238. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

