A portable, shock-proof, surface-heated droplet PCR system for Escherichia coli detection
- PMID: 26164008
- PMCID: PMC4549193
- DOI: 10.1016/j.bios.2015.06.026
A portable, shock-proof, surface-heated droplet PCR system for Escherichia coli detection
Abstract
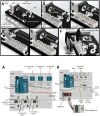
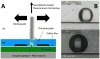
A novel polymerase chain reaction (PCR) device was developed that uses wire-guided droplet manipulation (WDM) to guide a droplet over three different heating chambers. After PCR amplification, end-point detection is achieved using a smartphone-based fluorescence microscope. The device was tested for identification of the 16S rRNA gene V3 hypervariable region from Escherichia coli genomic DNA. The lower limit of detection was 10(3) genome copies per sample. The device is portable with smartphone-based end-point detection and provides the assay results quickly (15 min for a 30-cycle amplification) and accurately. The system is also shock and vibration resistant, due to the multiple points of contact between the droplet and the thermocouple and the Teflon film on the heater surfaces. The thermocouple also provides real-time droplet temperature feedback to ensure it reaches the set temperature before moving to the next chamber/step in PCR. The device is equipped to use either silicone oil or coconut oil. Coconut oil provides additional portability and ease of transportation by eliminating spilling because its high melting temperature means it is solid at room temperature.
Keywords: 16S rRNA; Coconut oil; Contact angle; Polymerase chain reaction; Smartphone.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



References
-
- Angus SV, Kwon HJ, Yoon JY. J Environ Monit. 2012;14:3295–3304. - PubMed
-
- Baker M. Nat Meth. 2012;9:541–544.
-
- Bartlett JMS, Stirling D. PCR Protocols. 2nd. Humana Press; New Jersey: 2003.
-
- CDC (United States Centers for Disease Control and Prevention) Guidance for Public Health Laboratories on the Isolation and Characterization of Shiga Toxin-Producing Escherichia coli (STEC) from Clinical Specimens. [accessed 17.07.2014];2012 http://www.cdc.gov/ecoli/clinicians.html.
-
- Chang YH, Lee GB, Huang FC, Chen YY, Lin JL. Biomed Microdev. 2006;8:215–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

