In vivo electroretinographic studies of the role of GABAC receptors in retinal signal processing
- PMID: 26164072
- PMCID: PMC4573340
- DOI: 10.1016/j.exer.2015.07.002
In vivo electroretinographic studies of the role of GABAC receptors in retinal signal processing
Abstract
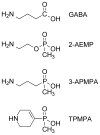
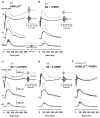
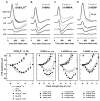
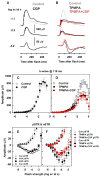
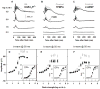
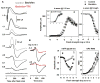
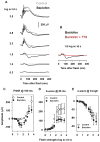
All three classes of receptors for the inhibitory neurotransmitter GABA (GABAR) are expressed in the retina. This study investigated roles of GABAR, especially GABACR (GABA(A)-ρ), in retinal signaling in vivo by studying effects on the mouse electroretinogram (ERG) of genetic deletion of GABACR versus pharmacological blockade using receptor antagonists. Brief full-field flash ERGs were recorded from anesthetized GABACR(-/-) mice, and WT C57BL/6 (B6) mice, before and after intravitreal injection of GABACR antagonists, TPMPA, 3-APMPA, or the more recently developed 2-AEMP; GABAAR antagonist, SR95531; GABABR antagonist, CGP, and agonist, baclofen. Intravitreal injections of TPMPA and SR95531 were also made in Brown Norway rats. The effect of 2-AEMP on GABA-induced current was tested directly in isolated rat rod bipolar cells, and 2-AEMP was found to preferentially block GABACR in those cells. Maximum amplitudes of dark (DA) and light-adapted (LA) ERG b-waves were reduced in GABACR(-/-) mice, compared to B6 mice, by 30-60%; a-waves were unaltered and oscillatory potential amplitudes were increased. In B6 mice, after injection of TPMPA (also in rats), 3-APMPA or 2-AEMP, ERGs became similar to ERGs of GABACR(-/-) mice. Blockade of GABAARs and GABABRs, or agonism of GABABRs did not alter B6 DA b-wave amplitude. The negative scotopic threshold response (nSTR) was slightly less sensitive in GABACR(-/-) than in B6 mice, and unaltered by 2-AEMP. However, amplitudes of nSTR and photopic negative response (PhNR), both of which originate from inner retina, were enhanced by TPMPA and 3-APMPA, each of which has GABAB agonist properties, and further increased by baclofen. The finding that genetic deletion of GABACR, the GABACR antagonist 2-AEMP, and other antagonists all reduced ERG b-wave amplitude, supports a role for GABACR in determining the maximum response amplitude of bipolar cells contributing to the b-wave. GABACR antagonists differed in their effects on nSTR and PhNR; antagonists with GABAB agonist properties enhanced light-driven responses whereas 2-AEMP did not.
Keywords: Electroretinogram; GABA; GABA receptors; Retina; Retinal signaling.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Modulation of the components of the rat dark-adapted electroretinogram by the three subtypes of GABA receptors.Vis Neurosci. 2003 Sep-Oct;20(5):535-42. doi: 10.1017/s0952523803205071. Vis Neurosci. 2003. PMID: 14977332
-
Voltage-gated sodium channels contribute to the b-wave of the rodent electroretinogram by mediating input to rod bipolar cell GABA(c) receptors.Exp Eye Res. 2013 Nov;116:279-90. doi: 10.1016/j.exer.2013.09.006. Epub 2013 Sep 21. Exp Eye Res. 2013. PMID: 24060343
-
Full-field electroretinogram in autism spectrum disorder.Doc Ophthalmol. 2016 Apr;132(2):83-99. doi: 10.1007/s10633-016-9529-y. Epub 2016 Feb 11. Doc Ophthalmol. 2016. PMID: 26868825
-
[Animal models of human retinal and optic nerve diseases analysed using electroretinography].Nippon Ganka Gakkai Zasshi. 2010 Mar;114(3):248-78, discussion 279. Nippon Ganka Gakkai Zasshi. 2010. PMID: 20387538 Review. Japanese.
-
Electroretinograms.Handb Clin Neurol. 2019;160:481-493. doi: 10.1016/B978-0-444-64032-1.00032-1. Handb Clin Neurol. 2019. PMID: 31277870 Review.
Cited by
-
Characterization of intraocular pressure pattern and changes of retinal ganglion cells in DBA2J glaucoma mice.Int J Ophthalmol. 2016 Feb 18;9(2):211-7. doi: 10.18240/ijo.2016.02.05. eCollection 2016. Int J Ophthalmol. 2016. PMID: 26949637 Free PMC article.
-
Subsequent and simultaneous electrophysiological investigation of the retina and the visual cortex in neurodegenerative and psychiatric diseases: what are the forecasts for the medicine of tomorrow?Front Psychiatry. 2023 Jun 2;14:1167654. doi: 10.3389/fpsyt.2023.1167654. eCollection 2023. Front Psychiatry. 2023. PMID: 37333926 Free PMC article. Review.
-
Contribution of GABAa, GABAc and glycine receptors to rat dark-adapted oscillatory potentials in the time and frequency domain.Oncotarget. 2017 Sep 8;8(44):77696-77709. doi: 10.18632/oncotarget.20770. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100418 Free PMC article.
-
Effects of medications on the human electroretinogram: A comprehensive review.Surv Ophthalmol. 2025 Apr 12:S0039-6257(25)00067-0. doi: 10.1016/j.survophthal.2025.04.003. Online ahead of print. Surv Ophthalmol. 2025. PMID: 40228746 Review.
-
Altered Visual Function in a Larval Zebrafish Knockout of Neurodevelopmental Risk Gene pdzk1.Invest Ophthalmol Vis Sci. 2021 Mar 1;62(3):29. doi: 10.1167/iovs.62.3.29. Invest Ophthalmol Vis Sci. 2021. PMID: 33749720 Free PMC article.
References
-
- Bush RA, Sieving PA. A proximal retinal component in the primate photopic erg a-wave. Invest Ophthalmol Vis Sci. 1994;35:635–645. - PubMed
-
- Cates LA, Li VS, Yakshe CC, Fadeyi MO, Andree TH, Karbon EW, et al. Phosphorus analogues of gamma-aminobutyric acid, a new class of anticonvulsants. Journal of Medicinal Chemistry. 1984;27:654–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

