Stress and Cocaine Trigger Divergent and Cell Type-Specific Regulation of Synaptic Transmission at Single Spines in Nucleus Accumbens
- PMID: 26164802
- PMCID: PMC4670821
- DOI: 10.1016/j.biopsych.2015.05.022
Stress and Cocaine Trigger Divergent and Cell Type-Specific Regulation of Synaptic Transmission at Single Spines in Nucleus Accumbens
Abstract
Background: Repeated exposure to cocaine or social stress leads to lasting structural and functional synaptic alterations in medium spiny neurons (MSNs) of nucleus accumbens (NAc). Although cocaine-induced and stress-induced structural changes in dendritic spines have been well documented, few studies have investigated functional consequences of cocaine and stress at the level of single spines.
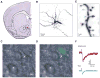
Methods: We exposed mice to chronic cocaine or chronic social defeat stress and used two-photon laser scanning microscopy with glutamate photo-uncaging and whole-cell recording to examine synaptic strength at individual spines on two distinct types of NAc MSNs in acute slices after 24 hours of cocaine withdrawal and after chronic social defeat stress.
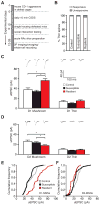
Results: In animals treated with cocaine, average synaptic strength was reduced specifically at large mushroom spines of MSNs expressing dopamine receptor type 1 (D1-MSNs). In contrast, cocaine promoted a rightward shift in the distribution of synaptic weights toward larger synaptic responses in MSNs expressing dopamine receptor type 2 (D2-MSNs). After chronic social defeat stress, resilient animals displayed an upregulation of synaptic strength at large mushroom spines of D1-MSNs and a concomitant downregulation in D2-MSNs. Although susceptible mice did not exhibit a significant overall change in synaptic strength on D1-MSNs or D2-MSNs, we observed a slight leftward shift in cumulative distribution of large synaptic responses in both cell types.
Conclusions: This study provides the first functional cell type-specific and spine type-specific comparison of synaptic strength at a single spine level between cocaine-induced and stress-induced neuroadaptations and demonstrates that psychoactive drugs and stress trigger divergent changes in synaptic function in NAc.
Keywords: Chronic social defeat stress; Cocaine addiction; Dendritic spine; Glutamate uncaging; Nucleus accumbens; Synaptic transmission.
Copyright © 2016 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
A NAc for Spinal Adjustments After Cocaine or Stress.Biol Psychiatry. 2016 Jun 1;79(11):872-4. doi: 10.1016/j.biopsych.2016.04.013. Biol Psychiatry. 2016. PMID: 27198520 Free PMC article. No abstract available.
References
-
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–72. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

