Comparative Meta-Analysis of Tenofovir Disoproxil Fumarate versus Emtricitabine and Tenofovir Disoproxil Fumarate as Treatments for Patients with Chronic Hepatitis B
- PMID: 26165204
- PMCID: PMC4499796
- DOI: 10.1038/srep11854
Comparative Meta-Analysis of Tenofovir Disoproxil Fumarate versus Emtricitabine and Tenofovir Disoproxil Fumarate as Treatments for Patients with Chronic Hepatitis B
Abstract
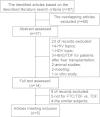
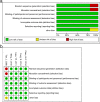
Tenofovir disoproxil fumarate (TDF) monotherapy has proven superior antiviral efficacy in chronic hepatitis B (CHB) patients; however, whether the combination of TDF and emtricitabine (FTC) exerts a significant advantage remains controversial. A meta-analysis was performed to comprehensively compare the therapeutic effects of FTC/TDF combination with TDF alone in CHB patients. Five studies involving 614 patients were identified, and subgroup analysis was performed based on the nucleos(t)ide treatment history. Our results revealed that in patients with nucleos(t)ide-naïve treatment, there were no significant differences between the treatment groups with TDF alone and FTC/TDF combination after 12 and 24 weeks; however, the FTC/TDF combination showed better viral suppression efficacy versus TDF alone after 48 (OR = 2.16, 95% CI = 1.06-4.41, P = 0.03), 96 (OR = 2.76, 95% CI = 1.29-5.92, P = 0.009) and 192 weeks (OR = 2.60, 95% CI = 1.21-5.56, P = 0.01). In patients with nucleos(t)ide treatment history, no differences were noted between the two treatment groups after 12, 24, 48 and 96 weeks. Our results indicated that FTC/TDF combination showed better viral suppression efficacy versus TDF alone in CHB patients with nucleos(t)ide-naïve treatment, while both treatments provided similar viral suppression efficacy in CHB patients with nucleos(t)ide treatment history.
Figures



Similar articles
-
Tenofovir disoproxil fumarate (TDF) vs. emtricitabine (FTC)/TDF in lamivudine resistant hepatitis B: A 5-year randomised study.J Hepatol. 2017 Jan;66(1):11-18. doi: 10.1016/j.jhep.2016.08.008. Epub 2016 Aug 18. J Hepatol. 2017. PMID: 27545497 Clinical Trial.
-
Tenofovir disoproxil fumarate monotherapy for nucleos(t)ide analogue-naïve and nucleos(t)ide analogue-experienced chronic hepatitis B patients.Clin Mol Hepatol. 2015 Mar;21(1):41-8. doi: 10.3350/cmh.2015.21.1.41. Epub 2015 Mar 25. Clin Mol Hepatol. 2015. PMID: 25834801 Free PMC article.
-
Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA.Gastroenterology. 2014 May;146(5):1240-8. doi: 10.1053/j.gastro.2014.01.044. Epub 2014 Jan 23. Gastroenterology. 2014. PMID: 24462735 Clinical Trial.
-
Comparative efficacy of tenofovir and entecavir in nucleos(t)ide analogue-naive chronic hepatitis B: A systematic review and meta-analysis.PLoS One. 2019 Nov 21;14(11):e0224773. doi: 10.1371/journal.pone.0224773. eCollection 2019. PLoS One. 2019. PMID: 31751366 Free PMC article.
-
HBeAg-negative chronic hepatitis B: why do I treat my patients with nucleos(t)ide analogues?Liver Int. 2014 Feb;34 Suppl 1:120-6. doi: 10.1111/liv.12401. Liver Int. 2014. PMID: 24373088 Review.
Cited by
-
Hepatitis B virus reverse transcriptase - Target of current antiviral therapy and future drug development.Antiviral Res. 2015 Nov;123:132-7. doi: 10.1016/j.antiviral.2015.09.011. Epub 2015 Sep 25. Antiviral Res. 2015. PMID: 26408354 Free PMC article. Review.
-
Tenofovir and adefovir down-regulate mitochondrial chaperone TRAP1 and succinate dehydrogenase subunit B to metabolically reprogram glucose metabolism and induce nephrotoxicity.Sci Rep. 2017 Apr 11;7:46344. doi: 10.1038/srep46344. Sci Rep. 2017. PMID: 28397817 Free PMC article.
-
Evidence of tenofovir resistance in chronic hepatitis B virus (HBV) infection: An observational case series of South African adults.J Clin Virol. 2020 Aug;129:104548. doi: 10.1016/j.jcv.2020.104548. Epub 2020 Jul 8. J Clin Virol. 2020. PMID: 32663786 Free PMC article.
References
-
- Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis 23, 47–58 (2003). - PubMed
-
- Ganem D. & Prince A. M. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med 350, 1118–1129 (2004). - PubMed
-
- World Health Organization. Hepatitis B (Updated March 2015). Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. Date of access: 26/03/2015.
-
- Si-Ahmed S. N. et al. Efficacy and tolerance of a combination of tenofovir disoproxil fumarate plus emtricitabine in patients with chronic hepatitis B: a European multicenter study. Antiviral research 92, 90–95 (2011). - PubMed
-
- EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 57, 167–185 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

