Mortality and loss to follow-up among HIV-infected persons on long-term antiretroviral therapy in Latin America and the Caribbean
- PMID: 26165322
- PMCID: PMC4499577
- DOI: 10.7448/IAS.18.1.20016
Mortality and loss to follow-up among HIV-infected persons on long-term antiretroviral therapy in Latin America and the Caribbean
Abstract
Introduction: Long-term survival of HIV patients after initiating highly active antiretroviral therapy (ART) has not been sufficiently described in Latin America and the Caribbean, as compared to other regions. The aim of this study was to describe the incidence of mortality, loss to follow-up (LTFU) and associated risk factors for patients enrolled in the Caribbean, Central and South America Network (CCASAnet).
Methods: We assessed time from ART initiation (baseline) to death or LTFU between 2000 and 2014 among ART-naïve adults (≥18 years) from sites in seven countries included in CCASAnet: Argentina, Brazil, Chile, Haiti, Honduras, Mexico and Peru. Kaplan-Meier techniques were used to estimate the probability of mortality over time. Risk factors for death were assessed using Cox regression models stratified by site and adjusted for sex, baseline age, nadir pre-ART CD4 count, calendar year of ART initiation, clinical AIDS at baseline and type of ART regimen.
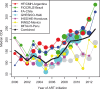
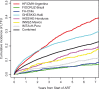
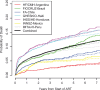
Results: A total of 16,996 ART initiators were followed for a median of 3.5 years (interquartile range (IQR): 1.6-6.2). The median age at ART initiation was 36 years (IQR: 30-44), subjects were predominantly male (63%), median CD4 count was 156 cells/µL (IQR: 60-251) and 26% of subjects had clinical AIDS prior to starting ART. Initial ART regimens were predominantly non-nucleoside reverse transcriptase inhibitor based (86%). The cumulative incidence of LTFU five years after ART initiation was 18.2% (95% confidence interval (CI) 17.5-18.8%). A total of 1582 (9.3%) subjects died; the estimated probability of death one, three and five years after ART initiation was 5.4, 8.3 and 10.3%, respectively. The estimated five-year mortality probability varied substantially across sites, from 3.5 to 14.0%. Risk factors for death were clinical AIDS at baseline (adjusted hazard ratio (HR)=1.65 (95% CI 1.47-1.87); p<0.001), lower baseline CD4 (HR=1.95 (95% CI 1.63-2.32) for 50 vs. 350 cells/µL; p<0.001) and older age (HR=1.47 (95% CI 1.29-1.69) for 50 vs. 30 years at ART initiation; p<0.001).
Conclusions: In this large, long-term study of mortality among HIV-positive adults initiating ART in Latin America and the Caribbean, overall estimates of mortality were heterogeneous, generally falling between those reported in high-income countries and sub-Saharan Africa.
Keywords: AIDS; ART; HIV; Latin America; long-term mortality; the Caribbean.
Figures

References
-
- UNAIDS. Geneva: UNAIDS; 2013. Global report: UNAIDS report on the global AIDS epidemic 2013.
-
- Wolff MJ, Cortes CP, Shepherd BE, Beltran CJ, Chilean ACSG. Long-term outcomes of a national expanded access program to antiretroviral therapy: the Chilean AIDS cohort. J Acquir Immune Defic Syndr. 2010;55(3):368–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

