GM130 Regulates Golgi-Derived Spindle Assembly by Activating TPX2 and Capturing Microtubules
- PMID: 26165940
- PMCID: PMC4506739
- DOI: 10.1016/j.cell.2015.06.014
GM130 Regulates Golgi-Derived Spindle Assembly by Activating TPX2 and Capturing Microtubules
Abstract
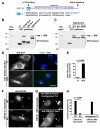
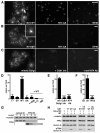
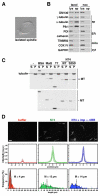
Spindle assembly requires the coordinated action of multiple cellular structures to nucleate and organize microtubules in a precise spatiotemporal manner. Among them, the contributions of centrosomes, chromosomes, and microtubules have been well studied, yet the involvement of membrane-bound organelles remains largely elusive. Here, we provide mechanistic evidence for a membrane-based, Golgi-derived microtubule assembly pathway in mitosis. Upon mitotic entry, the Golgi matrix protein GM130 interacts with importin α via a classical nuclear localization signal that recruits importin α to the Golgi membranes. Sequestration of importin α by GM130 liberates the spindle assembly factor TPX2, which activates Aurora-A kinase and stimulates local microtubule nucleation. Upon filament assembly, nascent microtubules are further captured by GM130, thus linking Golgi membranes to the spindle. Our results reveal an active role for the Golgi in regulating spindle formation to ensure faithful organelle inheritance.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Importin α phosphorylation promotes TPX2 activation by GM130 to control astral microtubules and spindle orientation.J Cell Sci. 2021 Feb 19;134(4):jcs258356. doi: 10.1242/jcs.258356. J Cell Sci. 2021. PMID: 33526712 Free PMC article.
-
Novel binding of the mitotic regulator TPX2 (target protein for Xenopus kinesin-like protein 2) to importin-alpha.J Biol Chem. 2010 Jun 4;285(23):17628-35. doi: 10.1074/jbc.M110.102343. Epub 2010 Mar 23. J Biol Chem. 2010. PMID: 20335181 Free PMC article.
-
Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts.Mol Biol Cell. 2004 Dec;15(12):5318-28. doi: 10.1091/mbc.e04-05-0385. Epub 2004 Sep 22. Mol Biol Cell. 2004. PMID: 15385625 Free PMC article.
-
The mechanism of spindle assembly: functions of Ran and its target TPX2.J Cell Biol. 2004 Sep 27;166(7):949-55. doi: 10.1083/jcb.200312112. J Cell Biol. 2004. PMID: 15452138 Free PMC article. Review.
-
Regulation of Aurora-A kinase on the mitotic spindle.Chromosoma. 2003 Dec;112(4):159-63. doi: 10.1007/s00412-003-0265-1. Epub 2003 Nov 21. Chromosoma. 2003. PMID: 14634755 Review.
Cited by
-
The Role of GM130 in Nervous System Diseases.Front Neurol. 2021 Oct 28;12:743787. doi: 10.3389/fneur.2021.743787. eCollection 2021. Front Neurol. 2021. PMID: 34777211 Free PMC article. Review.
-
Nucleation and Dynamics of Golgi-derived Microtubules.Front Neurosci. 2015 Nov 10;9:431. doi: 10.3389/fnins.2015.00431. eCollection 2015. Front Neurosci. 2015. PMID: 26617483 Free PMC article. Review.
-
Arf1 GTPase Regulates Golgi-Dependent G2/M Transition and Spindle Organization in Oocyte Meiosis.Adv Sci (Weinh). 2024 Jan;11(4):e2303009. doi: 10.1002/advs.202303009. Epub 2023 Nov 28. Adv Sci (Weinh). 2024. PMID: 38014604 Free PMC article.
-
GM130 regulates pulmonary surfactant protein secretion in alveolar type II cells.Sci China Life Sci. 2022 Jan;65(1):193-205. doi: 10.1007/s11427-020-1875-x. Epub 2021 Mar 16. Sci China Life Sci. 2022. PMID: 33740186
-
Mitotic spindle assembly in animal cells: a fine balancing act.Nat Rev Mol Cell Biol. 2017 Mar;18(3):187-201. doi: 10.1038/nrm.2016.162. Epub 2017 Feb 8. Nat Rev Mol Cell Biol. 2017. PMID: 28174430 Review.
References
-
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW. Flies without centrioles. Cell. 2006;125:1375–1386. - PubMed
-
- Bayliss R, Sardon T, Vernos I, Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell. 2003;12:851–862. - PubMed
-
- Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400:178–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

