Salvage Chemotherapy for Patients With Recurrent or Persistent Ovarian Clear Cell Carcinoma: A Retrospective Study of 164 Cases
- PMID: 26166110
- PMCID: PMC4504555
- DOI: 10.1097/MD.0000000000001121
Salvage Chemotherapy for Patients With Recurrent or Persistent Ovarian Clear Cell Carcinoma: A Retrospective Study of 164 Cases
Abstract
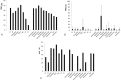
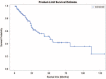
The purpose of this study was to evaluate the effects of salvage chemotherapy on recurrent or persistent ovarian clear cell carcinoma (CCC) with the goal of identifying a more rational treatment regimen for this lethal disease.The medical records of patients with CCC were retrospectively reviewed to select patients that were subsequently treated for recurrent or persistent disease.Of the 164 women with recurrent or persistent CCC, 485 chemotherapy courses with 1766 cycles were administered. Overall, the clinical benefit rate (CBR) was 39.4%, and the mean progression-free survival (PFS) was 4.5 months. Grade 3/4 toxicities occurred in 94 courses (19.4%). The CBR for TC was 45.1%, with a PFS of 3.7 months. Compared to that of TC, the CBRs for PC and CC were significantly lower (P = 0.020 and 0.021, respectively). The CBRs and PFS for PAF-C were slightly higher (P = 0.518 and 0.077, respectively), but showed a significantly higher adverse event rate (AER, P = 0.039). The CBR for bevacizumab was 50% with an extraordinarily long PFS (49.8 months). Gemcitabine and oxaliplatin had similar values for CBRs (44.4% and 44.1%) and PFS (2.5 and 3.4 months), respectively. Docetaxel (weekly) exhibited a notably low AER of 2.7%, and topotecan was associated with a relatively long PFS (7.7 months).For cis/carboplatin-pretreated patients, the existing active agents, such as oxaliplatin, gemcitabine, topotecan, and especially bevacizumab, are promising. Docetaxel (weekly) is well tolerated and might offer a particularly viable option for heavily pretreated patients. However, additional research to identify for a continued search for the optimal combination of chemotherapeutics or novel agents is still warranted.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
Similar articles
-
Salvage chemotherapy for recurrent or persistent clear cell carcinoma of the ovary: a single-institution experience for a series of 20 patients.Int J Clin Oncol. 2013 Feb;18(1):148-53. doi: 10.1007/s10147-011-0357-5. Epub 2011 Dec 10. Int J Clin Oncol. 2013. PMID: 22160560
-
Bevacizumab in recurrent ovarian cancer: could it be particularly effective in patients with clear cell carcinoma?Clin Transl Oncol. 2021 Mar;23(3):536-542. doi: 10.1007/s12094-020-02446-z. Epub 2020 Jul 10. Clin Transl Oncol. 2021. PMID: 32651885
-
Salvage chemotherapy in recurrent platinum-resistant or refractory epithelial ovarian cancer with Carboplatin and distearoylphosphatidylcholine pegylated liposomal Doxorubicin (lipo-dox®).Asian Pac J Cancer Prev. 2013;14(3):2131-5. doi: 10.7314/apjcp.2013.14.3.2131. Asian Pac J Cancer Prev. 2013. PMID: 23679331
-
Complete response to radiation therapy in a patient with chemotherapy-resistant ovarian clear cell adenocarcinoma.Arch Gynecol Obstet. 2002 Dec;267(2):98-100. doi: 10.1007/s00404-001-0258-3. Arch Gynecol Obstet. 2002. PMID: 12439556 Review.
-
Treatment of recurrent ovarian cancer.Ann Oncol. 2017 Nov 1;28(suppl_8):viii51-viii56. doi: 10.1093/annonc/mdx441. Ann Oncol. 2017. PMID: 29232464 Review.
Cited by
-
Bevacizumab in First-Line Chemotherapy Improves Progression-Free Survival for Advanced Ovarian Clear Cell Carcinoma.Cancers (Basel). 2021 Jun 25;13(13):3177. doi: 10.3390/cancers13133177. Cancers (Basel). 2021. PMID: 34202220 Free PMC article.
-
The prognostic value of pretreatment CA-125 levels and CA-125 normalization in ovarian clear cell carcinoma: a two-academic-institute study.Oncotarget. 2016 Mar 29;7(13):15566-76. doi: 10.18632/oncotarget.7216. Oncotarget. 2016. PMID: 26863639 Free PMC article.
-
Recurrence Patterns and Survival Outcomes in Chinese Patients with Surgically Treated Recurrent Ovarian Clear Cell Carcinoma: A Single Institutional Analysis of 45 Cases.Cancer Manag Res. 2020 Feb 7;12:913-919. doi: 10.2147/CMAR.S242129. eCollection 2020. Cancer Manag Res. 2020. PMID: 32104073 Free PMC article.
-
Clinicopathologic Significance of HNF-1β, AIRD1A, and PIK3CA Expression in Ovarian Clear Cell Carcinoma: A Tissue Microarray Study of 130 Cases.Medicine (Baltimore). 2016 Mar;95(9):e3003. doi: 10.1097/MD.0000000000003003. Medicine (Baltimore). 2016. PMID: 26945423 Free PMC article.
-
The post-progression survival of patients with recurrent or persistent ovarian clear cell carcinoma: results from a randomized phase III study in JGOG3017/GCIG.J Gynecol Oncol. 2020 Nov;31(6):e94. doi: 10.3802/jgo.2020.31.e94. J Gynecol Oncol. 2020. PMID: 33078599 Free PMC article. Clinical Trial.
References
-
- Pather S, Quinn MA. Clear-cell cancer of the ovary-is it chemosensitive? Int J Gynecol Cancer 2005; 15:432–437. - PubMed
-
- Chan JK, Teoh D, Hu JM, et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol 2008; 109:370–376. - PubMed
-
- Pectasides D, Fountzilas G, Aravantinos G, et al. Advanced stage clear-cell epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol Oncol 2006; 102:285–291. - PubMed
-
- Ho CM, Huang YJ, Chen TC, et al. Pure-type clear cell carcinoma of the ovary as a distinct histological type and improved survival in patients treated with paclitaxel-platinum-based chemotherapy in pure-type advanced disease. Gynecol Oncol 2004; 94:197–203. - PubMed
-
- Mahdi H, Moslemi-Kebria M, Levinson KL, et al. Prevalence and prognostic impact of lymphadenectomy and lymph node metastasis in clinically early-stage ovarian clear cell carcinoma. Int J Gynecol Cancer 2013; 23:1226–1230. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

