Efficacy of 4 Years of Octreotide Long-Acting Release Therapy in Patients With Severe Polycystic Liver Disease
- PMID: 26166166
- PMCID: PMC4928579
- DOI: 10.1016/j.mayocp.2015.05.011
Efficacy of 4 Years of Octreotide Long-Acting Release Therapy in Patients With Severe Polycystic Liver Disease
Abstract
Objective: To observe the effect on total liver volume (TLV) on and off therapy in selected symptomatic patients with autosomal dominant polycystic kidney disease (ADPKD) or autosomal dominant polycystic liver disease (PLD) who received octreotide long-acting release (OctLAR) for up to 4 years.
Patients and methods: Twenty-eight of 42 participants in a prospective 2-year clinical trial of OctLAR (40 mg monthly) consisting of double-blind, randomized (year 1) and open-label treatment (year 2) phases reenrolled in a 2-year open-label extension (OLE) study after being off OctLAR a mean of 8.3 months (original study: July 1, 2007, through June 30, 2013). Participants underwent magnetic resonance imaging at baseline, years 1 and 2, reenrollment, and study completion. Primary end point: change in TLV; secondary end points: changes in total kidney volume, glomerular filtration rate, quality of life (QoL), safety, vital signs, and laboratory parameters.
Results: Twenty-five participants (59.5%) completed the OLE. Off therapy, TLVs increased a mean ± SD of 3.4%±8.2% per year; after resuming therapy, TLVs decreased a mean ± SD of -4.7%±6.1% per year. Despite regrowth off treatment, overall reductions were observed, with a median (interquartile range) TLV of 4047 mL (3107-7402 mL) at baseline and 3477 (2653-7131 mL) at study completion (-13.2%; P<.001) and with improved health-related QoL. Total kidney volumes increased, and glomerular filtration rates declined from 58.2 mL/min to 54.5 mL/min (n=16) in patients with ADPKD on therapy from baseline to study completion.
Conclusion: Therapy with OctLAR over 4 years in selected patients with symptomatic PLD arrested PLD progression, alleviating symptoms and improving health-related QoL. Discontinuation led to organ regrowth.
Trial registration: clinicaltrials.gov Identifier: NCT00426153.
Copyright © 2015 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures
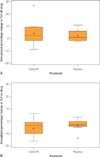

References
-
- Bae KT, Zhu F, Chapman AB, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol. 2006;1(1):64–69. - PubMed
-
- Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68(1):206–216. - PubMed
-
- van Keimpema L, Nevens F, Vanslembrouck R, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137(5):1661–1668. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

