The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells
- PMID: 26166259
- PMCID: PMC7110702
- DOI: 10.1016/j.pupt.2015.07.001
The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells
Abstract
Background: Serine proteases act through the proteolytic cleavage of the hemagglutinin (HA) of influenza viruses for the entry of influenza virus into cells, resulting in infection. However, the inhibitory effects of serine protease inhibitors on influenza virus infection of human airway epithelial cells, and on their production of inflammatory cytokines are unclear.
Methods: Primary cultures of human tracheal epithelial cells were treated with four types of serine protease inhibitors, including camostat, and infected with A/Sendai-H/108/2009/(H1N1) pdm09 or A/New York/55/2004(H3N2).
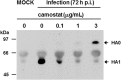
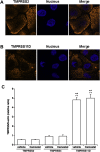
Results: Camostat reduced the amounts of influenza viruses in the supernatants and viral RNA in the cells. It reduced the cleavage of an influenza virus precursor protein, HA0, into the subunit HA1. Camostat also reduced the concentrations of the cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α in the supernatants. Gabexate and aprotinin reduced the viral titers and RNA levels in the cells, and aprotinin reduced the concentrations of TNF-α in the supernatants. The proteases transmembrane protease serine S1 member (TMPRSS) 2 and HAT (human trypsin-like protease: TMPRSS11D), which are known to cleave HA0 and to activate the virus, were detected at the cell membrane and in the cytoplasm. mRNA encoding TMPRSS2, TMPRSS4 and TMPRSS11D was detectable in the cells, and the expression levels were not affected by camostat.
Conclusions: These findings suggest that human airway epithelial cells express these serine proteases and that serine protease inhibitors, especially camostat, may reduce influenza viral replication and the resultant production of inflammatory cytokines possibly through inhibition of activities of these proteases.
Keywords: Airway epithelial cell; Camostat; Cell culture; Influenza; Interleukin; Serine protease.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

References
-
- Hayden F.G., Gwaltney J.M., Jr. Viral infections. In: Murray J., Nadel J.A., editors. Textbook of Respiratory Medicine. Saunders Co; Philadelphia: 1988. pp. 748–802.
-
- Perez-Padilla R., de la Rosa-Zamboni D., Ponce de Leon S., Hernandez M., Quinones-Falconi F., Bautista E. INER Working Group on influenza, Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N. Engl. J. Med. 2009;361:680–689. - PubMed
-
- Minor T.E., Dick E.C., Baker J.W., Ouellette J.J., Cohen M., Reed C.E. Rhinovirus and influenza type A infections as precipitants of asthma. Am. Rev. Respir. Dis. 1976;113:149–153. - PubMed
-
- Nichol K.L., Margolis K.L., Wuorenma J., Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N. Engl. J. Med. 1994;331:778–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

