Noninvasive Imaging of Nanomedicines and Nanotheranostics: Principles, Progress, and Prospects
- PMID: 26166537
- PMCID: PMC4610064
- DOI: 10.1021/cr500314d
Noninvasive Imaging of Nanomedicines and Nanotheranostics: Principles, Progress, and Prospects
Figures



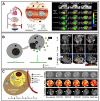


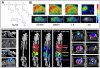





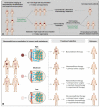
References
-
- European Science Foundation’s Forward Look Nanomedicine: An EMRC Consensus Opinion. European Science Foundation; 2005. www.esf.org.
-
- Kataoka K, Harada A, Nagasaki Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Deliv. Rev. 2001;47:113–131. - PubMed
-
- Panyam J, Labhasetwar V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003;55:329–347. - PubMed
-
- Torchilin VP. Recent Advances With Liposomes As Pharmaceutical Carriers. Nat. Rev. Drug Discovery. 2005;4:145–160. - PubMed
-
- Duncan R. Polymer Conjugates As Anticancer Nanomedicines. Nat. Rev. Cancer. 2006;6:688–701. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

