Aurora A Kinase Contributes to a Pole-Based Error Correction Pathway
- PMID: 26166783
- PMCID: PMC4509859
- DOI: 10.1016/j.cub.2015.06.021
Aurora A Kinase Contributes to a Pole-Based Error Correction Pathway
Abstract
Chromosome biorientation, where sister kinetochores attach to microtubules (MTs) from opposing spindle poles, is the configuration that best ensures equal partitioning of the genome during cell division. Erroneous kinetochore-MT attachments are commonplace but are often corrected prior to anaphase. Error correction, thought to be mediated primarily by the centromere-enriched Aurora B kinase (ABK), typically occurs near spindle poles; however, the relevance of this locale is unclear. Furthermore, polar ejection forces (PEFs), highest near poles, can stabilize improper attachments by pushing mal-oriented chromosome arms away from spindle poles. Hence, there is a conundrum: erroneous kinetochore-MT attachments are weakened where PEFs are most likely to strengthen them. Here, we report that Aurora A kinase (AAK) opposes the stabilizing effect of PEFs. AAK activity contributes to phosphorylation of kinetochore substrates near poles and its inhibition results in chromosome misalignment and an increased incidence of erroneous kinetochore-MT attachments. Furthermore, AAK directly phosphorylates a site in the N-terminal tail of Ndc80/Hec1 that has been implicated in reducing the affinity of the Ndc80 complex for MTs when phosphorylated. We propose that an AAK activity gradient contributes to correcting mal-oriented kinetochore-MT attachments in the vicinity of spindle poles.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
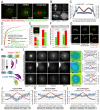
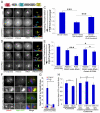
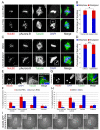
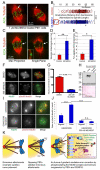
Comment in
-
Chromosome Segregation: A Spatial Code to Correct Kinetochore-Microtubule Attachments.Curr Biol. 2015 Jul 20;25(14):R601-3. doi: 10.1016/j.cub.2015.05.056. Curr Biol. 2015. PMID: 26196485
References
-
- Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci. 2003;116:4213–4225. - PubMed
-
- Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568–581. - PubMed
-
- Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. - PubMed
-
- Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

