Synthesis of fused uracils: pyrano[2,3- d]pyrimidines and 1,4-bis(pyrano[2,3- d]pyrimidinyl)benzenes by domino Knoevenagel/Diels-Alder reactions
- PMID: 26166870
- PMCID: PMC4495049
- DOI: 10.1007/s00706-012-0781-x
Synthesis of fused uracils: pyrano[2,3- d]pyrimidines and 1,4-bis(pyrano[2,3- d]pyrimidinyl)benzenes by domino Knoevenagel/Diels-Alder reactions
Abstract
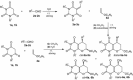
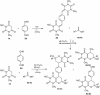
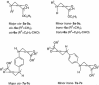
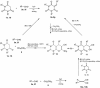
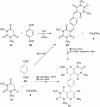
Abstract: Knoevenagel condensation of barbituric acids with aromatic aldehydes containing one or two formyl groups was carried out. 5-Arylidenebarbituric acids underwent smooth hetero-Diels-Alder (HDA) reactions with enol ethers to afford cis and trans diastereoisomers of pyrano[2,3-d]pyrimidine-2,4-diones and 5,5'-(1,4-phenylene)bis[2H-pyrano[2,3-d]pyrimidine-2,4(3H)-dione] derivatives in excellent yields (75-88 %). Syntheses were realized by Knoevenagel condensation and HDA reaction in four different reaction conditions: Knoevenagel condensation in water and Diels-Alder reaction in methylene chloride solution, Knoevenagel condensation in water and Diels-Alder reaction without solvent, three-component one-pot reaction in methylene chloride solution, or three-component one-pot reaction in water. All reactions were carried out without catalyst at room temperature. The reactions of malononitrile with Knoevenagel condensation products of barbituric acids and heteroaromatic aldehydes or terephthalaldehyde were examined and did not provide corresponding pyranopyrimidines.
Keywords: Cycloadditions; Drug research; Michael addition; One-pot synthesis.
Figures
Similar articles
-
Recent Advances in Inverse-Electron-Demand Hetero-Diels-Alder Reactions of 1-Oxa-1,3-Butadienes.Top Curr Chem (Cham). 2016 Jun;374(3):24. doi: 10.1007/s41061-016-0026-2. Epub 2016 Apr 20. Top Curr Chem (Cham). 2016. PMID: 27573264 Free PMC article. Review.
-
An efficient synthesis of novel pyrano[2,3-d]- and furopyrano[2,3-d]pyrimidines via indium-catalyzed multi-component domino reaction.Beilstein J Org Chem. 2006 Jun 13;2:11. doi: 10.1186/1860-5397-2-11. Beilstein J Org Chem. 2006. PMID: 16768808 Free PMC article.
-
Towards organo-click chemistry: development of organocatalytic multicomponent reactions through combinations of aldol, Wittig, Knoevenagel, Michael, Diels-Alder and Huisgen cycloaddition reactions.Chemistry. 2004 Oct 25;10(21):5323-31. doi: 10.1002/chem.200400597. Chemistry. 2004. PMID: 15390208
-
4,4'-trimethylenedipiperidine as a nitrogen heterocycle solvent and/or catalyst: Liquid phase tandem Knoevenagel-Michael condensation.Turk J Chem. 2021 Feb 17;45(1):261-268. doi: 10.3906/kim-2010-41. eCollection 2021. Turk J Chem. 2021. PMID: 33679168 Free PMC article.
-
[The chemistry of pericyclic reactions and their application to syntheses of heterocyclic compounds].Yakugaku Zasshi. 2003 Sep;123(9):717-59. doi: 10.1248/yakushi.123.717. Yakugaku Zasshi. 2003. PMID: 14513766 Review. Japanese.
Cited by
-
An experimental and mechanism study on the regioselective click reaction toward the synthesis of thiazolidinone-triazole.Heliyon. 2021 Feb 2;7(2):e06113. doi: 10.1016/j.heliyon.2021.e06113. eCollection 2021 Feb. Heliyon. 2021. PMID: 33644441 Free PMC article.
-
Synthesis, physico-chemical characterization, and environmental applications of meso porous crosslinked poly (azomethine-sulfone)s.Sci Rep. 2022 Jul 27;12(1):12878. doi: 10.1038/s41598-022-17042-0. Sci Rep. 2022. PMID: 35896584 Free PMC article.
-
Recent Advances in Inverse-Electron-Demand Hetero-Diels-Alder Reactions of 1-Oxa-1,3-Butadienes.Top Curr Chem (Cham). 2016 Jun;374(3):24. doi: 10.1007/s41061-016-0026-2. Epub 2016 Apr 20. Top Curr Chem (Cham). 2016. PMID: 27573264 Free PMC article. Review.
-
Importance of Hybrid Catalysts toward the Synthesis of 5H-Pyrano[2,3-d]pyrimidine-2-ones/2,4-diones (Thiones).ACS Omega. 2023 Jan 3;8(2):1759-1816. doi: 10.1021/acsomega.2c05349. eCollection 2023 Jan 17. ACS Omega. 2023. PMID: 36687108 Free PMC article. Review.
References
-
- Boger DL, Weinreb SN. Hetero Diels-Alder methodology in organic synthesis. San Diego: Academic Press; 1987.
-
- Tietze LF, Kettschau G. Top Curr Chem. 1997;189:12.
-
- Kitamura N, Ohnishi A (1984) Eur Pat 163599; Chem Abstr 104:186439u
-
- Furuja S, Ohtaki T (1994) Chem Abstr 121:205395w. Eur Pat Appl EP 608565
-
- Heber D, Heers C, Ravens U. Pharmazie. 1993;48:537. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous