Magnetic Nanoparticles for Early Detection of Cancer by Magnetic Resonance Imaging
- PMID: 26166945
- PMCID: PMC4495966
- DOI: 10.1557/mrs2009.120
Magnetic Nanoparticles for Early Detection of Cancer by Magnetic Resonance Imaging
Abstract
This article provides a brief overview of recent progress in the synthesis and functionalization of magnetic nanoparticles and their applications in the early detection of malignant tumors by magnetic resonance imaging (MRI). The intrinsic low sensitivity of MRI necessitates the use of large quantities of exogenous contrast agents in many imaging studies. Magnetic nanoparticles have recently emerged as highly efficient MRI contrast agents because these nanometer-scale materials can carry high payloads while maintaining the ability to move through physiological systems. Superparamagnetic ferrite nanoparticles (such as iron oxide) provide excellent negative contrast enhancement. Recent refinement of synthetic methodologies has led to ferrite nanoparticles with narrow size distributions and high crystallinity. Target-specific tumor imaging becomes possible through functionalization of ferrite nanoparticles with targeting agents to allow for site-specific accumulation. Nanoparticulate contrast agents capable of positive contrast enhancement have recently been developed in order to overcome the drawbacks of negative contrast enhancement afforded by ferrite nanoparticles. These newly developed magnetic nanoparticles have the potential to enable physicians to diagnose cancer at the earliest stage possible and thus can have an enormous impact on more effective cancer treatment.
Figures







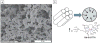



References
-
- Stark DD, Bradley WG., Jr . Magnetic Resonance Imaging. Mosby; St. Louis: 1999.
-
- Aime S, Botta M, Fasano M, Terreno E. Chem Soc Rev. 1998;27:19.
-
- Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293. - PubMed
-
- Larson SM. Cancer. 1991;67(Suppl. 4):1253. - PubMed
-
- Yang DJ, Kim EE, Inoue T. Ann Nucl Med. 2006;20:1. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
