Clinical predictors of thiopurine-related adverse events in Crohn's disease
- PMID: 26167079
- PMCID: PMC4491966
- DOI: 10.3748/wjg.v21.i25.7795
Clinical predictors of thiopurine-related adverse events in Crohn's disease
Abstract
Aim: To determine the incidence and predictors of thiopurine-related adverse events.
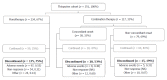
Methods: Subjects with Crohn's disease who were followed in the Alberta Inflammatory Bowel Disease Consortium patient database registry were identified. Retrospective chart review was conducted between August 5th, 2010 and June 1st, 2012. We collected data on: age at diagnosis; sex; disease location and behaviour at time of prescribing thiopurine; perianal fistulising disease at or prior to thiopurine prescription; smoking status at time of thiopurine prescription, use of corticosteroid within 6 mo of diagnosis; dosage, age at onset, and cessation of 5-aminosalicyclic acid (5-ASA); anti-tumour necrosis factor medication exposure and intestinal resection before thiopurine prescription. The primary outcome of interest was the first adverse event that led to discontinuation of the first thiopurine medication used. Logistic regression models were used to associate clinical characteristics with outcomes after adjusting for potential confounders. Risk estimates were presented as odds ratios (OR) with 95% CI. Effect modification by age and sex were explored.
Results: Our cohort had a median follow-up duration of 5.8 years [interquartile range (IQR 25th-75th) 2.7-9.1]. Thiopurine therapy was discontinued in 31.3% of patients because of: hypersensitivity reactions (7.1%), acute pancreatitis (6.2%), gastrointestinal intolerance (5.4%), leucopenia (3.7%), hepatotoxicity (3.4%), infection (1.1%) and other reasons (4.3%). A higher incidence of thiopurine withdrawal was observed in patients over the age of 40 (39.4%, P = 0.007). A sex-by-age interaction (P = 0.04) was observed. Females older than 40 years of age had an increased risk of thiopurine discontinuation due to an adverse event (age above 40 vs age below 40, adjusted OR = 2.8; 95%CI: 1.4-5.6). In contrast, age did not influence thiopurine withdrawal in males (age above 40 vs below 40, adjusted OR = 0.9; 95%CI: 0.4-2.1). Other clinical variables (disease location and phenotype, perianal disease, smoking history, history of intestinal resection and prior 5-ASA or corticosteroid use) were not associated with an increased risk an adverse event leading to therapy cessation.
Conclusion: Thiopurine withdrawal due to adverse events is commoner in women over the age of 40 at prescription. These findings need to be replicated in other cohorts.
Keywords: Adverse events; Azathioprine; Mercaptopurine; Thiopurines.
Figures
References
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. - PubMed
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. - PubMed
-
- Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. - PubMed
-
- Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn‘s disease. Cochrane Database Syst Rev. 2010;(6):CD000545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical