Innovative methods in soil phosphorus research: A review
- PMID: 26167132
- PMCID: PMC4497464
- DOI: 10.1002/jpln.201400327
Innovative methods in soil phosphorus research: A review
Abstract
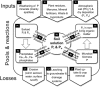
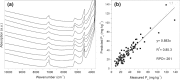
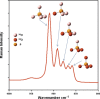
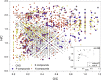
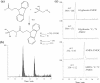
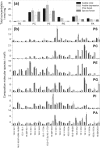
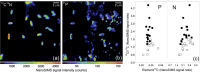
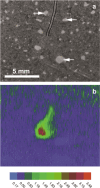
Phosphorus (P) is an indispensable element for all life on Earth and, during the past decade, concerns about the future of its global supply have stimulated much research on soil P and method development. This review provides an overview of advanced state-of-the-art methods currently used in soil P research. These involve bulk and spatially resolved spectroscopic and spectrometric P speciation methods (1 and 2D NMR, IR, Raman, Q-TOF MS/MS, high resolution-MS, NanoSIMS, XRF, XPS, (µ)XAS) as well as methods for assessing soil P reactions (sorption isotherms, quantum-chemical modeling, microbial biomass P, enzymes activity, DGT, 33P isotopic exchange, 18O isotope ratios). Required experimental set-ups and the potentials and limitations of individual methods present a guide for the selection of most suitable methods or combinations.
Keywords: nutrient cycling; phosphate; soil chemistry; speciation; spectroscopy.
Figures




References
-
- Abdi D, Tremblay GF, Ziadi N, Bélanger G, Parent LÉ. Predicting soil phosphorus-related properties using reflectance spectroscopy. Soil Sci. Soc. Am. J. 2012;76:2318–2326.
-
- Abdulla HAN, Sleighter RL, Hatcher PG. Two dimensional correlation analysis of Fourier transform ion cyclotron resonance mass spectra of dissolved organic matter: A new graphical analysis of trends. Anal. Chem. 2013;85:3895–3902. - PubMed
-
- Acelas NY, Mejia SM, Mondragón F, Flórez E. Density functional theory characterization of phosphate and sulfate adsorption on Fe-(hydr)oxide: Reactivity, pH effect, estimation of Gibbs free energies, and topological analysis of hydrogen bonds. Comp. Theor. Chem. 2013;1005:16–24.
-
- Achat DL, Bakker MR, Zeller B, Pellerin S, Bienaimé S, Morel C. Long-term organic phosphorus mineralization in Spodosols under forests and its relation to carbon and nitrogen mineralization. Soil Biol. Biochem. 2010;42:1479–149.
-
- Addiscott TM, Brockie D, Catt JA, Christian DG, Harris GL, Howse KR, Mirza NA, Pepper TJ. Phosphate losses through field drains in a heavy cultivated soil. J. Environ. Qual. 2000;29:522–532.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
