A Novel Matrine Derivative WM130 Inhibits Activation of Hepatic Stellate Cells and Attenuates Dimethylnitrosamine-Induced Liver Fibrosis in Rats
- PMID: 26167476
- PMCID: PMC4488526
- DOI: 10.1155/2015/203978
A Novel Matrine Derivative WM130 Inhibits Activation of Hepatic Stellate Cells and Attenuates Dimethylnitrosamine-Induced Liver Fibrosis in Rats
Abstract
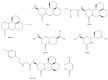
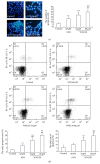
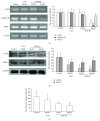
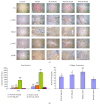
Activation of hepatic stellate cells (HSCs) is a critical event in process of hepatic fibrogenesis and cirrhosis. Matrine, the active ingredient of Sophora, had been used for clinical treatment of acute/chronic liver disease. However, its potency was low. We prepared a high potency and low toxicity matrine derivate, WM130 (C30N4H40SO5F), which exhibited better pharmacological activities on antihepatic fibrosis. This study demonstrated that WM130 results in a decreased proliferative activity of HSC-T6 cells, with the half inhibitory concentration (IC50) of 68 μM. WM130 can inhibit the migration and induce apoptosis in HSC-T6 cells at both concentrations of 68 μM (IC50) and 34 μM (half IC50). The expression of α-SMA, Collagen I, Collagen III, and TGF-β1 could be downregulated, and the protein phosphorylation levels of EGFR, AKT, ERK, Smad, and Raf (p-EGFR, p-AKT, p-ERK, p-Smad, and p-Raf) were also decreased by WM130. On the DMN-induced rat liver fibrosis model, WM130 can effectively reduce the TGF-β1, AKT, α-SMA, and p-ERK levels, decrease the extracellular matrix (ECM) formation, and inhibit rat liver fibrosis progression. In conclusion, this study demonstrated that WM130 can significantly inhibit the activation of HSC-T6 cells and block the rat liver fibrosis progression by inducing apoptosis, suppressing the deposition of ECM, and inhibiting TGF-β/Smad and Ras/ERK pathways.
Figures



References
-
- Hu L., Peng X., Tang Y., Liu Y. Synthesis of peptides of Carapax Trionycis and their inhibitory effects on TGF-β1-induced hepatic stellate cells. Drug Discoveries & Therapeutics. 2013;7(6):248–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

