Photoactivatable genetically encoded calcium indicators for targeted neuronal imaging
- PMID: 26167640
- PMCID: PMC4597790
- DOI: 10.1038/nmeth.3480
Photoactivatable genetically encoded calcium indicators for targeted neuronal imaging
Abstract
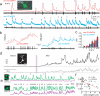
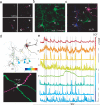
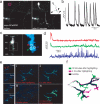
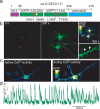
Circuit mapping requires knowledge of both structural and functional connectivity between cells. Although optical tools have been made to assess either the morphology and projections of neurons or their activity and functional connections, few probes integrate this information. We have generated a family of photoactivatable genetically encoded Ca(2+) indicators that combines attributes of high-contrast photolabeling with high-sensitivity Ca(2+) detection in a single-color protein sensor. We demonstrated in cultured neurons and in fruit fly and zebrafish larvae how single cells could be selected out of dense populations for visualization of morphology and high signal-to-noise measurements of activity, synaptic transmission and connectivity. Our design strategy is transferrable to other sensors based on circularly permutated GFP (cpGFP).
Figures


References
-
- Cajal SR. Estructura de los centros nerviosos de las aves. Revista Trimestral de Histología Normal y Patológica. 1888;1:1–10.
-
- Chung K, Deisseroth K. CLARITY for mapping the nervous system. Nat. Methods. 2013;10:508–13. - PubMed
-
- Livet J, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

