Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation
- PMID: 26168074
- PMCID: PMC9003232
- DOI: 10.1021/acsnano.5b03162
Piezoelectric Nanoparticle-Assisted Wireless Neuronal Stimulation
Abstract
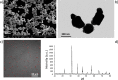
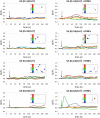
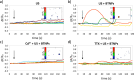
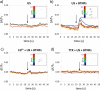
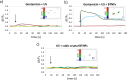
Tetragonal barium titanate nanoparticles (BTNPs) have been exploited as nanotransducers owing to their piezoelectric properties, in order to provide indirect electrical stimulation to SH-SY5Y neuron-like cells. Following application of ultrasounds to cells treated with BTNPs, fluorescence imaging of ion dynamics revealed that the synergic stimulation is able to elicit a significant cellular response in terms of calcium and sodium fluxes; moreover, tests with appropriate blockers demonstrated that voltage-gated membrane channels are activated. The hypothesis of piezoelectric stimulation of neuron-like cells was supported by lack of cellular response in the presence of cubic nonpiezoelectric BTNPs, and further corroborated by a simple electroelastic model of a BTNP subjected to ultrasounds, according to which the generated voltage is compatible with the values required for the activation of voltage-sensitive channels.
Keywords: SH-SY5Y cells; barium titanate nanoparticles; calcium imaging; piezoelectricity; ultrasounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

