Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism
- PMID: 26168401
- PMCID: PMC5086585
- DOI: 10.1038/nature14587
Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism
Abstract
Activation of cellular stress response pathways to maintain metabolic homeostasis is emerging as a critical growth and survival mechanism in many cancers. The pathogenesis of pancreatic ductal adenocarcinoma (PDA) requires high levels of autophagy, a conserved self-degradative process. However, the regulatory circuits that activate autophagy and reprogram PDA cell metabolism are unknown. Here we show that autophagy induction in PDA occurs as part of a broader transcriptional program that coordinates activation of lysosome biogenesis and function, and nutrient scavenging, mediated by the MiT/TFE family of transcription factors. In human PDA cells, the MiT/TFE proteins--MITF, TFE3 and TFEB--are decoupled from regulatory mechanisms that control their cytoplasmic retention. Increased nuclear import in turn drives the expression of a coherent network of genes that induce high levels of lysosomal catabolic function essential for PDA growth. Unbiased global metabolite profiling reveals that MiT/TFE-dependent autophagy-lysosome activation is specifically required to maintain intracellular amino acid pools. These results identify the MiT/TFE proteins as master regulators of metabolic reprogramming in pancreatic cancer and demonstrate that transcriptional activation of clearance pathways converging on the lysosome is a novel hallmark of aggressive malignancy.
Figures
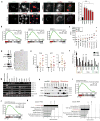
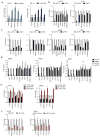



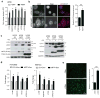
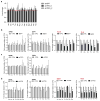






Comment in
-
Autophagy: Surviving stress in pancreatic cancer.Nat Rev Cancer. 2015 Sep;15(9):513. doi: 10.1038/nrc4005. Nat Rev Cancer. 2015. PMID: 26299587 No abstract available.
References
-
- Yang A, et al. Autophagy Is Critical for Pancreatic Tumor Growth and Progression in Tumors with p53 Alterations. Cancer Discov. 2014;4:905–913. doi: 10.1158/2159-8290.CD-14-0362. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

