Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases
- PMID: 26168713
- PMCID: PMC4501059
- DOI: 10.1038/srep11827
Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases
Erratum in
-
Corrigendum: Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases.Sci Rep. 2015 Sep 18;5:14157. doi: 10.1038/srep14157. Sci Rep. 2015. PMID: 26383116 Free PMC article. No abstract available.
Abstract
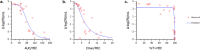
With the diminishing effectiveness of current antibacterial therapies, it is critically important to discover agents that operate by a mechanism that circumvents existing resistance. ETX0914, the first of a new class of antibacterial agent targeted for the treatment of gonorrhea, operates by a novel mode-of-inhibition against bacterial type II topoisomerases. Incorporating an oxazolidinone on the scaffold mitigated toxicological issues often seen with topoisomerase inhibitors. Organisms resistant to other topoisomerase inhibitors were not cross-resistant with ETX0914 nor were spontaneous resistant mutants to ETX0914 cross-resistant with other topoisomerase inhibitor classes, including the widely used fluoroquinolone class. Preclinical evaluation of ETX0914 pharmacokinetics and pharmacodynamics showed distribution into vascular tissues and efficacy in a murine Staphylococcus aureus infection model that served as a surrogate for predicting efficacious exposures for the treatment of Neisseria gonorrhoeae infections. A wide safety margin to the efficacious exposure in toxicological evaluations supported progression to Phase 1. Dosing ETX0914 in human volunteers showed sufficient exposure and minimal adverse effects to expect a highly efficacious anti-gonorrhea therapy.
Conflict of interest statement
The authors are or have been employed by AstraZeneca and declare no competing financial interests otherwise.
Figures
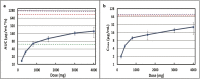
References
-
- Rodvolk K. A. & McConeghy K. W. Methicillin-resistant Staphylococcus aureus therapy: past, present and future. Clin. Infect. Dis. 58, S20–S27 (2014). - PubMed
-
- Diacon A. H. et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360, 2397–2405 (2009). - PubMed
-
- Ruble J. C. et al. Synthesis of (-)-PNU-286607 by asymmetric cyclization of alkylidene barbiturates. J. Am. Chem. Soc. 131, 3991–3997 (2009). - PubMed
-
- Basarab G. S. et al. Novel DNA gyrase inhibiting spiropyrimidinetriones with a benzisoxazole scaffold – SAR and in vivo characterization. J. Med. Chem. 57, 9078–9095 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

