Goal-directed Fluid Therapy May Improve Hemodynamic Stability of Parturient with Hypertensive Disorders of Pregnancy Under Combined Spinal Epidural Anesthesia for Cesarean Delivery and the Well-being of Newborns
- PMID: 26168834
- PMCID: PMC4717919
- DOI: 10.4103/0366-6999.160546
Goal-directed Fluid Therapy May Improve Hemodynamic Stability of Parturient with Hypertensive Disorders of Pregnancy Under Combined Spinal Epidural Anesthesia for Cesarean Delivery and the Well-being of Newborns
Abstract
Background: Hypotension induced by combined spinal epidural anesthesia in parturient with hypertensive disorders of pregnancy (HDP) can easily compromise blood supply to vital organs including uteroplacental perfusion and result in fetal distress. The aim of this study was to investigate whether the goal-directed fluid therapy (GDFT) with LiDCO rapid system can improve well-being of both HDP parturient and their babies.
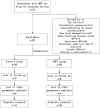
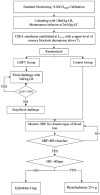
Methods: Fifty-two stable HDP parturient scheduled for elective cesarean delivery were recruited. After loading with 10 ml/kg lactated Ringer's solution (LR), parturient were randomized to the GDFT and control group. In the GDFT group, individualized fluid therapy was guided by increase in stroke volume (ΔSV) provided via LiDCO rapid system. The control group received the routine fluid therapy. The primary endpoints included maternal hypotension and the doses of vasopressors administered prior to fetal delivery. The secondary endpoints included umbilical blood gas abnormalities and neonatal adverse events.
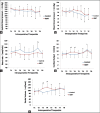
Results: The severity of HDP was similar between two groups. The total LR infusion (P < 0.01) and urine output (P < 0.05) were higher in the GDFT group than in the control group. Following twice fluid challenge tests, the systolic blood pressure, mean blood pressure, cardiac output and SV in the GDFT group were significantly higher, and the heart rate was lower than in the control group. The incidence of maternal hypotension and doses of phenylephrine used prior to fetal delivery were significantly higher in the control group than in the GDFT group (P < 0.01). There were no differences in the Apgar scores between two groups. In the control group, the mean values of pH in umbilical artery/vein were remarkably decreased (P < 0.05), and the incidences of neonatal hypercapnia and hypoxemia were statistically increased (P < 0.05) than in the GDFT group.
Conclusions: Dynamic responsiveness guided fluid therapy with the LiDCO rapid system may provide potential benefits to stable HDP parturient and their babies.
Conflict of interest statement
Figures
References
-
- Dennis AT. Management of pre-eclampsia: Issues for anaesthetists. Anaesthesia. 2012;67:1009–20. - PubMed
-
- Ferrazzani S, Luciano R, Garofalo S, D’Andrea V, De Carolis S, De Carolis MP, et al. Neonatal outcome in hypertensive disorders of pregnancy. Early Hum Dev. 2011;87:445–9. - PubMed
-
- Barra S, Cachulo Mdo C, Providência R, Leitão-Marques A. Hypertension in pregnancy: The current state of the art. Rev Port Cardiol. 2012;31:425–32. - PubMed
-
- Singh R, Kumar N, Jain A, Chakraborty M. Spinal anesthesia for lower segment Cesarean section in patients with stable eclampsia. J Clin Anesth. 2011;23:202–6. - PubMed
-
- Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: An endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

