In vivo analysis of Nef's role in HIV-1 replication, systemic T cell activation and CD4(+) T cell loss
- PMID: 26169178
- PMCID: PMC4501112
- DOI: 10.1186/s12977-015-0187-z
In vivo analysis of Nef's role in HIV-1 replication, systemic T cell activation and CD4(+) T cell loss
Abstract
Background: Nef is a multifunctional HIV-1 protein critical for progression to AIDS. Humans infected with nef(-) HIV-1 have greatly delayed or no disease consequences. We have contrasted nef(-) and nef(+) infection of BLT humanized mice to better characterize Nef's pathogenic effects.
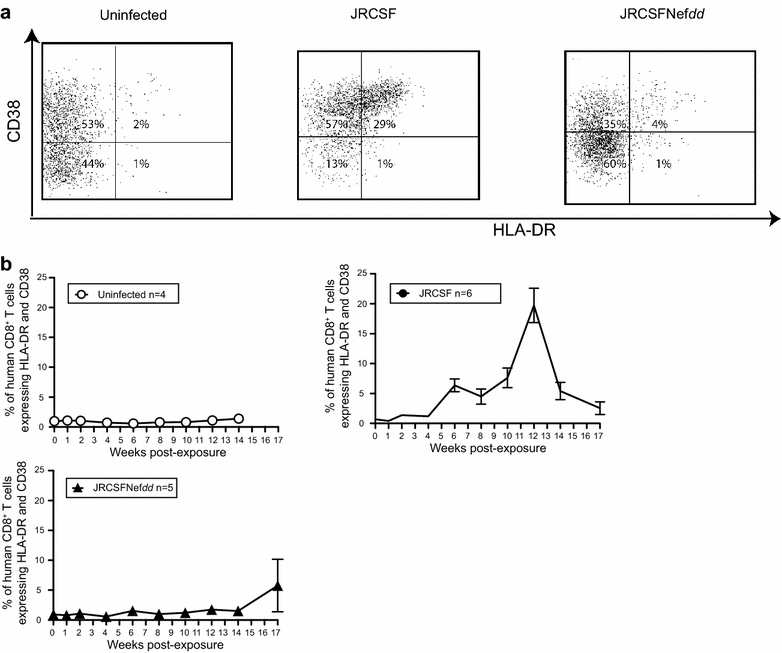
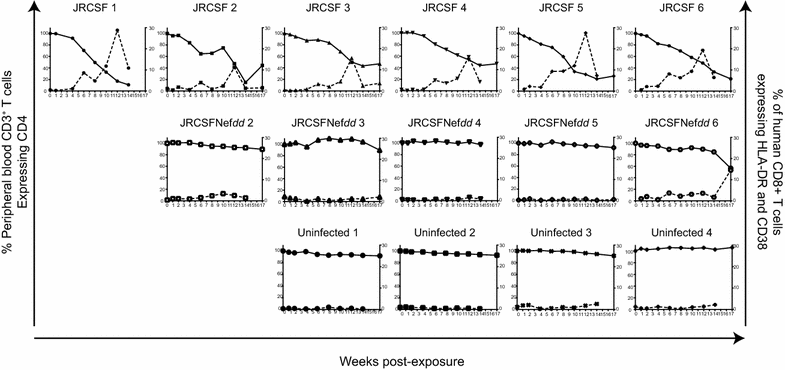
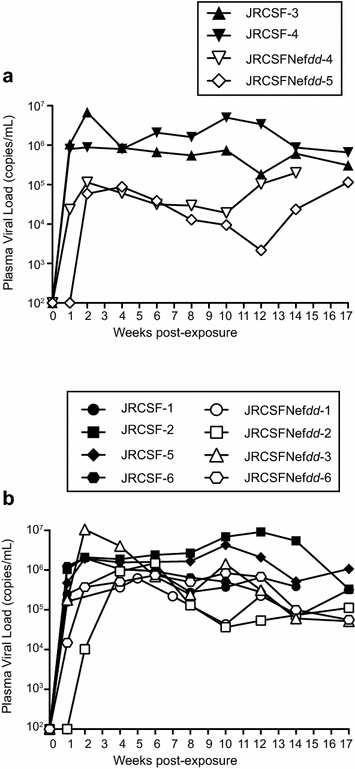
Results: Mice were inoculated with CCR5-tropic HIV-1JRCSF (JRCSF) or JRCSF with an irreversibly inactivated nef (JRCSFNefdd). In peripheral blood (PB), JRCSF exhibited high levels of viral RNA (peak viral loads of 4.71 × 10(6) ± 1.23 × 10(6) copies/ml) and a progressive, 75% loss of CD4(+) T cells over 17 weeks. Similar losses were observed in CD4(+) T cells from bone marrow, spleen, lymph node, lung and liver but thymocytes were not significantly decreased. JRCSFNefdd also had high peak viral loads (2.31 × 10(6) ± 1.67 × 10(6)) but induced no loss of PB CD4(+) T cells. In organs, JRCSFNefdd produced small, but significant, reductions in CD4(+) T cell levels and did not affect the level of thymocytes. Uninfected mice have low levels of HLA-DR(+)CD38(+)CD8(+) T cells in blood (1-2%). Six weeks post inoculation, JRCSF infection resulted in significantly elevated levels of activated CD8(+) T cells (6.37 ± 1.07%). T cell activation coincided with PB CD4(+) T cell loss which suggests a common Nef-dependent mechanism. At 12 weeks, in JRCSF infected animals PB T cell activation sharply increased to 19.7 ± 2.9% then subsided to 5.4 ± 1.4% at 14 weeks. HLA-DR(+)CD38(+)CD8(+) T cell levels in JRCSFNefdd infected mice did not rise above 1-2% despite sustained high levels of viremia. Interestingly, we also noted that in mice engrafted with human tissue expressing a putative protective HLA-B allele (B42:01), JRCSFNefdd exhibited a substantial (200-fold) reduced viral load compared to JRCSF.
Conclusions: Nef expression was necessary for both systemic T cell activation and substantial CD4(+) T cell loss from blood and tissues. JRCSFNefdd infection did not activate CD8(+) T cells or reduce the level of CD4(+) T cells in blood but did result in a small Nef-independent decrease in CD4(+) T cells in organs. These observations strongly support the conclusion that viral pathogenicity is mostly driven by Nef. We also observed for the first time substantial host-specific suppression of HIV-1 replication in a small animal infection model.
Figures



Similar articles
-
Nef functions in BLT mice to enhance HIV-1 replication and deplete CD4+CD8+ thymocytes.Retrovirology. 2012 May 28;9:44. doi: 10.1186/1742-4690-9-44. Retrovirology. 2012. PMID: 22640559 Free PMC article.
-
Human endothelial cells enhance human immunodeficiency virus type 1 replication in CD4+ T cells in a Nef-dependent manner in vitro and in vivo.J Virol. 2005 Jan;79(1):264-76. doi: 10.1128/JVI.79.1.264-276.2005. J Virol. 2005. PMID: 15596822 Free PMC article.
-
HIV-1 Nef and Vpu Interfere with L-Selectin (CD62L) Cell Surface Expression To Inhibit Adhesion and Signaling in Infected CD4+ T Lymphocytes.J Virol. 2015 May;89(10):5687-700. doi: 10.1128/JVI.00611-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25822027 Free PMC article.
-
Modelling thymic HIV-1 Nef effects.Curr HIV Res. 2006 Jan;4(1):57-64. doi: 10.2174/157016206775197583. Curr HIV Res. 2006. PMID: 16454711 Review.
-
Mechanism of HIV pathogenesis: role of superantigens in disease.Alcohol Clin Exp Res. 1998 Aug;22(5 Suppl):188S-192S. doi: 10.1097/00000374-199805001-00001. Alcohol Clin Exp Res. 1998. PMID: 9727632 Review.
Cited by
-
Innate Immune Reconstitution in Humanized Bone Marrow-Liver-Thymus (HuBLT) Mice Governs Adaptive Cellular Immune Function and Responses to HIV-1 Infection.Front Immunol. 2021 May 26;12:667393. doi: 10.3389/fimmu.2021.667393. eCollection 2021. Front Immunol. 2021. PMID: 34122425 Free PMC article.
-
In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection.Retrovirology. 2016 May 21;13(1):36. doi: 10.1186/s12977-016-0268-7. Retrovirology. 2016. PMID: 27206407 Free PMC article.
-
Bioengineering extracellular vesicle cargo for optimal therapeutic efficiency.Mol Ther Methods Clin Dev. 2024 Apr 26;32(2):101259. doi: 10.1016/j.omtm.2024.101259. eCollection 2024 Jun 13. Mol Ther Methods Clin Dev. 2024. PMID: 38770107 Free PMC article. Review.
-
APOBEC3G and APOBEC3F Act in Concert To Extinguish HIV-1 Replication.J Virol. 2016 Apr 14;90(9):4681-4695. doi: 10.1128/JVI.03275-15. Print 2016 May. J Virol. 2016. PMID: 26912618 Free PMC article.
-
Tight-Binding Hydroxypyrazole HIV-1 Nef Inhibitors Suppress Viral Replication in Donor Mononuclear Cells and Reverse Nef-Mediated MHC-I Downregulation.ACS Infect Dis. 2020 Feb 14;6(2):302-312. doi: 10.1021/acsinfecdis.9b00382. Epub 2019 Dec 16. ACS Infect Dis. 2020. PMID: 31775511 Free PMC article.
References
-
- Calugi G, Montella F, Favalli C, Benedetto A. Entire genome of a strain of human immunodeficiency virus type 1 with a deletion of nef that was recovered 20 years after primary infection: large pool of proviruses with deletions of env. J Virol. 2006;80:11892–11896. doi: 10.1128/JVI.00932-06. - DOI - PMC - PubMed
-
- Kondo M, Shima T, Nishizawa M, Sudo K, Iwamuro S, Okabe T, et al. Identification of attenuated variants of HIV-1 circulating recombinant form 01_AE that are associated with slow disease progression due to gross genetic alterations in the nef/long terminal repeat sequences. J Infect Dis. 2005;192:56–61. doi: 10.1086/430739. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

