How Much Are Biosimilars Used in Clinical Practice? A Retrospective Italian Population-Based Study of Erythropoiesis-Stimulating Agents in the Years 2009-2013
- PMID: 26169209
- PMCID: PMC4561997
- DOI: 10.1007/s40259-015-0132-7
How Much Are Biosimilars Used in Clinical Practice? A Retrospective Italian Population-Based Study of Erythropoiesis-Stimulating Agents in the Years 2009-2013
Abstract
Purpose: To explore the prescription patterns of erythropoiesis-stimulating agents (ESAs) in four large Italian geographic areas, where different health policy interventions to promote biosimilar use in routine care are undertaken.
Methods: A retrospective drug utilization study was conducted during the years 2009-2013. The data sources were the administrative databases of the Tuscany region and of the Caserta, Palermo, and Treviso Local Health Units (LHUs). The characteristics, prevalence, and switching patterns of different ESAs (biosimilars and reference products), stratified by indication for use, were calculated over time and across centers.
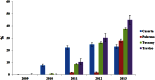
Results: Overall, 49,491 patients were treated with ESAs during the years 2009-2013 in the four centers. Of these, 41,286 patients (83.4 %) were naive users. The prevalence of ESA use increased from 2.9 to 3.4 per 1000 inhabitants in the years 2009-2011 but decreased thereafter (3.0 per 1000 in 2013). Moreover, the proportion of biosimilar users increased overall from 1.8 % in 2010 to 33.6 % in 2013, with larger increase in Treviso (from 0.0 to 45.0 %) and Tuscany (from 0.7 to 37.6 %) than in Caserta (from 7.5 to 22.9 %) and Palermo (from 0.0 to 27.7 %). Switching between different ESAs during the first year of therapy was frequent (17.0 %), much more toward reference products than toward biosimilars.
Conclusion: Overall, the prevalence of ESA use decreased slightly, while use of biosimilar ESAs, especially in naive patients, increased significantly but to different extents in these four large Italian geographic areas. Switching between different ESAs during the first year of treatment was very frequent, which may affect pharmacovigilance monitoring. New strategies are necessary to further improve market penetration of low-cost medicines, such as biosimilars, and also to harmonize effective health policy interventions that aim to reduce pharmaceutical expenses and optimize patient benefit across all regions.
Figures



References
-
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP): guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin....
-
- Agenzia Italiana del Farmaco (AIFA). Position paper sui farmaci biosimilari (28/05/2013). Available from: http://www.agenziafarmaco.gov.it/sites/default/files/AIFA_POSITION_PAPER....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

