Inhibition of AAC(6')-Ib-mediated resistance to amikacin in Acinetobacter baumannii by an antisense peptide-conjugated 2',4'-bridged nucleic acid-NC-DNA hybrid oligomer
- PMID: 26169414
- PMCID: PMC4538503
- DOI: 10.1128/AAC.01304-15
Inhibition of AAC(6')-Ib-mediated resistance to amikacin in Acinetobacter baumannii by an antisense peptide-conjugated 2',4'-bridged nucleic acid-NC-DNA hybrid oligomer
Abstract
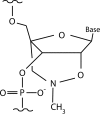
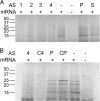
Multiresistant Acinetobacter baumannii, a common etiologic agent of severe nosocomial infections in compromised hosts, usually harbors aac(6')-Ib. This gene specifies resistance to amikacin and other aminoglycosides, seriously limiting the effectiveness of these antibiotics. An antisense oligodeoxynucleotide (ODN4) that binds to a duplicated sequence on the aac(6')-Ib mRNA, one of the copies overlapping the initiation codon, efficiently inhibited translation in vitro. An isosequential nuclease-resistant hybrid oligomer composed of 2',4'-bridged nucleic acid-NC (BNA(NC)) residues and deoxynucleotides (BNA(NC)-DNA) conjugated to the permeabilizing peptide (RXR)4XB ("X" and "B" stand for 6-aminohexanoic acid and β-alanine, respectively) (CPPBD4) inhibited translation in vitro at the same levels observed in testing ODN4. Furthermore, CPPBD4 in combination with amikacin inhibited growth of a clinical A. baumannii strain harboring aac(6')-Ib in liquid cultures, and when both compounds were used as combination therapy to treat infected Galleria mellonella organisms, survival was comparable to that seen with uninfected controls.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Restoration of susceptibility to amikacin by 8-hydroxyquinoline analogs complexed to zinc.PLoS One. 2019 May 29;14(5):e0217602. doi: 10.1371/journal.pone.0217602. eCollection 2019. PLoS One. 2019. PMID: 31141575 Free PMC article.
-
In Vitro and In Vivo Activity of a Novel Antisense Peptide Nucleic Acid Compound Against Multidrug-Resistant Acinetobacter baumannii.Microb Drug Resist. 2019 Sep;25(7):961-965. doi: 10.1089/mdr.2018.0179. Epub 2019 Apr 22. Microb Drug Resist. 2019. PMID: 31009322
-
External guide sequences targeting the aac(6')-Ib mRNA induce inhibition of amikacin resistance.Antimicrob Agents Chemother. 2007 Jun;51(6):1918-25. doi: 10.1128/AAC.01500-06. Epub 2007 Mar 26. Antimicrob Agents Chemother. 2007. PMID: 17387154 Free PMC article.
-
Inhibition of aminoglycoside 6'-N-acetyltransferase type Ib by zinc: reversal of amikacin resistance in Acinetobacter baumannii and Escherichia coli by a zinc ionophore.Antimicrob Agents Chemother. 2014 Jul;58(7):4238-41. doi: 10.1128/AAC.00129-14. Epub 2014 May 12. Antimicrob Agents Chemother. 2014. PMID: 24820083 Free PMC article.
-
Genetic basis of resistance to aminoglycosides in Acinetobacter spp. and spread of armA in Acinetobacter baumannii sequence group 1 in Korean hospitals.Diagn Microbiol Infect Dis. 2009 Jun;64(2):185-90. doi: 10.1016/j.diagmicrobio.2009.02.010. Epub 2009 Apr 9. Diagn Microbiol Infect Dis. 2009. PMID: 19361944
Cited by
-
Promising strategies employing nucleic acids as antimicrobial drugs.Mol Ther Nucleic Acids. 2024 Jan 18;35(1):102122. doi: 10.1016/j.omtn.2024.102122. eCollection 2024 Mar 12. Mol Ther Nucleic Acids. 2024. PMID: 38333674 Free PMC article. Review.
-
How CRISPR-Cas System Could Be Used to Combat Antimicrobial Resistance.Infect Drug Resist. 2020 Apr 20;13:1111-1121. doi: 10.2147/IDR.S247271. eCollection 2020. Infect Drug Resist. 2020. PMID: 32368102 Free PMC article. Review.
-
Evaluation of Aminoglycoside and Carbapenem Resistance in a Collection of Drug-Resistant Pseudomonas aeruginosa Clinical Isolates.Microb Drug Resist. 2018 Sep;24(7):1020-1030. doi: 10.1089/mdr.2017.0101. Epub 2017 Dec 20. Microb Drug Resist. 2018. PMID: 29261405 Free PMC article.
-
Galleria mellonella as a Good Model to Study Acinetobacter baumannii Pathogenesis.Pathogens. 2021 Nov 14;10(11):1483. doi: 10.3390/pathogens10111483. Pathogens. 2021. PMID: 34832638 Free PMC article. Review.
-
Can Vitamin B12 Assist the Internalization of Antisense LNA Oligonucleotides into Bacteria?Antibiotics (Basel). 2021 Apr 3;10(4):379. doi: 10.3390/antibiotics10040379. Antibiotics (Basel). 2021. PMID: 33916701 Free PMC article.
References
-
- Peleg AY, de Breij A, Adams MD, Cerqueira GM, Mocali S, Galardini M, Nibbering PH, Earl AM, Ward DV, Paterson DL, Seifert H, Dijkshoorn L. 2012. The success of Acinetobacter species; genetic, metabolic and virulence attributes. PLoS One 7:e46984. doi:10.1371/journal.pone.0046984. - DOI - PMC - PubMed
-
- Hartstein AI, Rashad AL, Liebler JM, Actis LA, Freeman J, Rourke JW Jr, Stibolt TB, Tolmasky ME, Ellis GR, Crosa JH. 1988. Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am J Med 85:624–631. doi:10.1016/S0002-9343(88)80233-X. - DOI - PubMed
-
- Bai H, You Y, Yan H, Meng J, Xue X, Hou Z, Zhou Y, Ma X, Sang G, Luo X. 2012. Antisense inhibition of gene expression and growth in gram-negative bacteria by cell-penetrating peptide conjugates of peptide nucleic acids targeted to rpoD gene. Biomaterials 33:659–667. doi:10.1016/j.biomaterials.2011.09.075. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

