An HNF1α-regulated feedback circuit modulates hepatic fibrogenesis via the crosstalk between hepatocytes and hepatic stellate cells
- PMID: 26169608
- PMCID: PMC4528057
- DOI: 10.1038/cr.2015.84
An HNF1α-regulated feedback circuit modulates hepatic fibrogenesis via the crosstalk between hepatocytes and hepatic stellate cells
Abstract
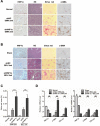
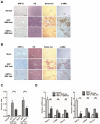
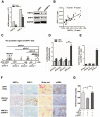
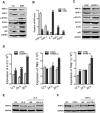
Hepatocytes are critical for the maintenance of liver homeostasis, but its involvement in hepatic fibrogenesis remains elusive. Hepatocyte nuclear factor 1α (HNF1α) is a liver-enriched transcription factor that plays a key role in hepatocyte function. Our previous study revealed a significant inhibitory effect of HNF1α on hepatocellular carcinoma. In this study, we report that the expression of HNF1α is significantly repressed in both human and rat fibrotic liver. Knockdown of HNF1α in the liver significantly aggravates hepatic fibrogenesis in either dimethylnitrosamine (DMN) or bile duct ligation (BDL) model in rats. In contrast, forced expression of HNF1α markedly alleviates hepatic fibrosis. HNF1α regulates the transcriptional expression of SH2 domain-containing phosphatase-1 (SHP-1) via directly binding to SHP-1 promoter in hepatocytes. Inhibition of SHP-1 expression abrogates the anti-fibrotic effect of HNF1α in DMN-treated rats. Moreover, HNF1α repression in primary hepatocytes leads to the activation of NF-κB and JAK/STAT pathways and initiates an inflammatory feedback circuit consisting of HNF1α, SHP-1, STAT3, p65, miR-21 and miR-146a, which sustains the deregulation of HNF1α in hepatocytes. More interestingly, a coordinated crosstalk between hepatocytes and hepatic stellate cells (HSCs) participates in this positive feedback circuit and facilitates the progression of hepatocellular damage. Our findings demonstrate that impaired hepatocytes play an active role in hepatic fibrogenesis. Early intervention of HNF1α-regulated inflammatory feedback loop in hepatocytes may have beneficial effects in the treatment of chronic liver diseases.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

