Detecting Germline PTEN Mutations Among At-Risk Patients With Cancer: An Age- and Sex-Specific Cost-Effectiveness Analysis
- PMID: 26169622
- PMCID: PMC4525048
- DOI: 10.1200/JCO.2014.60.3456
Detecting Germline PTEN Mutations Among At-Risk Patients With Cancer: An Age- and Sex-Specific Cost-Effectiveness Analysis
Abstract
Purpose: Cowden syndrome (CS) is an autosomal dominant disorder characterized by benign and malignant tumors. One-quarter of patients who are diagnosed with CS have pathogenic germline PTEN mutations, which increase the risk of the development of breast, thyroid, uterine, renal, and other cancers. PTEN testing and regular, intensive cancer surveillance allow for early detection and treatment of these cancers for mutation-positive patients and their relatives. Individual CS-related features, however, occur commonly in the general population, making it challenging for clinicians to identify CS-like patients to offer PTEN testing.
Patients and methods: We calculated the cost per mutation detected and analyzed the cost-effectiveness of performing selected PTEN testing among CS-like patients using a semi-quantitative score (the PTEN Cleveland Clinic [CC] score) compared with existing diagnostic criteria. In our model, first-degree relatives of the patients with detected PTEN mutations are offered PTEN testing. All individuals with detected PTEN mutations are offered cancer surveillance.
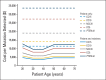
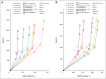
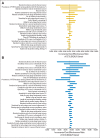
Results: CC score at a threshold of 15 (CC15) costs from $3,720 to $4,573 to detect one PTEN mutation, which is the most inexpensive among the different strategies. At base-case, CC10 is the most cost-effective strategy for female patients who are younger than 40 years, and CC15 is the most cost-effective strategy for female patients who are between 40 and 60 years of age and male patients of all ages. In sensitivity analyses, CC15 is robustly the most cost-effective strategy for probands who are younger than 60 years.
Conclusion: Use of the CC score as a clinical risk calculator is a cost-effective prescreening method to identify CS-like patients for PTEN germline testing.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



Similar articles
-
Germline PTEN Mutation Analysis for PTEN Hamartoma Tumor Syndrome.Methods Mol Biol. 2016;1388:63-73. doi: 10.1007/978-1-4939-3299-3_6. Methods Mol Biol. 2016. PMID: 27033071
-
Breast cancer risk and clinical implications for germline PTEN mutation carriers.Breast Cancer Res Treat. 2017 Aug;165(1):1-8. doi: 10.1007/s10549-015-3665-z. Epub 2015 Dec 23. Breast Cancer Res Treat. 2017. PMID: 26700035 Review.
-
PTEN hamartoma tumor syndrome: clinical risk assessment and management protocol.Methods. 2015 May;77-78:11-9. doi: 10.1016/j.ymeth.2014.10.011. Epub 2014 Oct 22. Methods. 2015. PMID: 25461771 Review.
-
PTEN germline mutations in patients initially tested for other hereditary cancer syndromes: would use of risk assessment tools reduce genetic testing?Oncologist. 2013;18(10):1083-90. doi: 10.1634/theoncologist.2013-0174. Epub 2013 Sep 13. Oncologist. 2013. PMID: 24037976 Free PMC article.
-
Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes.Am J Hum Genet. 2008 Aug;83(2):261-8. doi: 10.1016/j.ajhg.2008.07.011. Am J Hum Genet. 2008. PMID: 18678321 Free PMC article.
Cited by
-
A systematic review of the methodological quality of economic evaluations in genetic screening and testing for monogenic disorders.Genet Med. 2022 Feb;24(2):262-288. doi: 10.1016/j.gim.2021.10.008. Epub 2021 Dec 7. Genet Med. 2022. PMID: 34906467 Free PMC article.
-
Outcomes of contralateral prophylactic mastectomy in relation to familial history: a decision analysis (BRCR-D-16-00033).Breast Cancer Res. 2016 Sep 20;18(1):93. doi: 10.1186/s13058-016-0752-y. Breast Cancer Res. 2016. PMID: 27650678 Free PMC article.
-
Potentially pathogenic germline CHEK2 c.319+2T>A among multiple early-onset cancer families.Fam Cancer. 2018 Jan;17(1):141-153. doi: 10.1007/s10689-017-0011-0. Fam Cancer. 2018. PMID: 28608266
-
Economic evaluations of predictive genetic testing: A scoping review.PLoS One. 2023 Aug 2;18(8):e0276572. doi: 10.1371/journal.pone.0276572. eCollection 2023. PLoS One. 2023. PMID: 37531363 Free PMC article.
-
Evaluation of Patients and Families With Concern for Predispositions to Hematologic Malignancies Within the Hereditary Hematologic Malignancy Clinic (HHMC).Clin Lymphoma Myeloma Leuk. 2016 Jul;16(7):417-428.e2. doi: 10.1016/j.clml.2016.04.001. Epub 2016 Apr 27. Clin Lymphoma Myeloma Leuk. 2016. PMID: 27210295 Free PMC article.
References
-
- US Department of Health and Human Services. Washington, DC: Office of Disease Prevention and Health Promotion; Healthy People 2020. www.healthypeople.gov/2020/topics-objectives/topic/genomics.
-
- American Cancer Society. Cancer Facts & Figures 2014. http://www.cancer.org/Research/CancerFactsStatistics/CancerFactsFigures2....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

